Copper depletion modulates mitochondrial oxidative phosphorylation to impair triple negative breast cancer metastasis
- PMID: 34911956
- PMCID: PMC8674260
- DOI: 10.1038/s41467-021-27559-z
Copper depletion modulates mitochondrial oxidative phosphorylation to impair triple negative breast cancer metastasis
Abstract
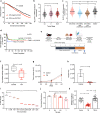
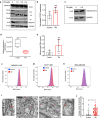
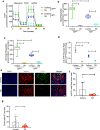
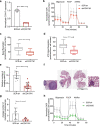
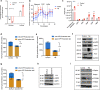
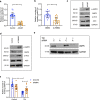
Copper serves as a co-factor for a host of metalloenzymes that contribute to malignant progression. The orally bioavailable copper chelating agent tetrathiomolybdate (TM) has been associated with a significant survival benefit in high-risk triple negative breast cancer (TNBC) patients VSports手机版. Despite these promising data, the mechanisms by which copper depletion impacts metastasis are poorly understood and this remains a major barrier to advancing TM to a randomized phase II trial. Here, using two independent TNBC models, we report a discrete subpopulation of highly metastatic SOX2/OCT4+ cells within primary tumors that exhibit elevated intracellular copper levels and a marked sensitivity to TM. Global proteomic and metabolomic profiling identifies TM-mediated inactivation of Complex IV as the primary metabolic defect in the SOX2/OCT4+ cell population. We also identify AMPK/mTORC1 energy sensor as an important downstream pathway and show that AMPK inhibition rescues TM-mediated loss of invasion. Furthermore, loss of the mitochondria-specific copper chaperone, COX17, restricts copper deficiency to mitochondria and phenocopies TM-mediated alterations. These findings identify a copper-metabolism-metastasis axis with potential to enrich patient populations in next-generation therapeutic trials. .
Trial registration: ClinicalTrials. gov NCT00195091. V体育安卓版.
© 2021. The Author(s).
Conflict of interest statement (VSports手机版)
The authors declare no competing interests.
Figures

References
-
- Malhotra MK, Emens LA. The evolving management of metastatic triple negative breast cancer. Semin. Oncol. 2020;47:229–237. - "V体育官网" PubMed
-
- Fouani L, Menezes SV, Paulson M, Richardson DR, Kovacevic Z. Metals and metastasis: exploiting the role of metals in cancer metastasis to develop novel anti-metastatic agents. Pharm. Res. 2017;115:275–287. - "VSports最新版本" PubMed
-
- Weiss KH, et al. WTX101 in patients newly diagnosed with Wilson disease: final results of a global, prospective phase 2 trial. J. Hepatol. 2017;66:S88.
"VSports app下载" Publication types
MeSH terms
- VSports手机版 - Actions
- "V体育官网" Actions
- "V体育官网入口" Actions
- V体育官网入口 - Actions
- V体育官网 - Actions
- Actions (V体育ios版)
- "VSports在线直播" Actions
- Actions (V体育官网)
- "V体育2025版" Actions
- "VSports" Actions
- V体育安卓版 - Actions
- "VSports注册入口" Actions
- "VSports最新版本" Actions
VSports注册入口 - Substances
- Actions (V体育官网入口)
- VSports注册入口 - Actions
- "V体育ios版" Actions
- "V体育2025版" Actions
- "VSports最新版本" Actions
- "V体育平台登录" Actions
- VSports - Actions
Associated data
- Actions (VSports最新版本)
VSports - Grants and funding
LinkOut - more resources
Full Text Sources
Medical

