V体育2025版 - Naringin Exerts Therapeutic Effects on Mice Colitis: A Study Based on Transcriptomics Combined With Functional Experiments
- PMID: 34504431
- PMCID: PMC8421552 (VSports)
- DOI: 10.3389/fphar.2021.729414
Naringin Exerts Therapeutic Effects on Mice Colitis: A Study Based on Transcriptomics Combined With Functional Experiments
Abstract
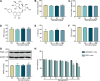
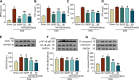
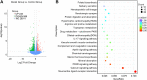
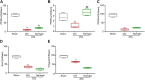
Naringin has been shown to exert protective effects in an animal model of ulcerative colitis, but detailed mechanisms remain unclear. This study aimed to investigate function and signaling mechanisms underlying naringin-induced therapeutic effects on colitis. Two mouse models were established to mimic human Inflammatory bowel disease (IBD) by treating drinking water with dextran sodium sulphate or intra-colonic administration of 2, 4, 6-trinitrobenzene sulfonic acid. Transcriptomics combined with functional experiments were used to investigate underlying mechanisms. Colitis symptoms, including weight loss and high disease activity index were significantly reversed by naringin. The inflammatory response, oxidative reactions, and epithelial cell apoptosis that occur with colitis were also alleviated by naringin. After naringin treatment, transcriptomics results identified 753 differentially expressed mRNAs that were enriched in signaling pathways, including the neuroactive ligand-receptor interaction, calcium signaling, and peroxisome proliferator-activated receptor (PPAR) signaling VSports手机版. The naringin-induced alleviation of colitis was significantly inhibited by the PPAR-γ inhibitor BADGE. In IEC-6 and RAW264. 7 cells incubated with lipopolysaccharide (LPS), NF-κB-p65, a downstream protein of PPAR-γ, was significantly increased. Naringin suppressed LPS-induced high expression of NF-κB-p65, which was inhibited by small interfering RNA targeting PPAR-γ. Our study clarifies detailed mechanisms underlying naringin-induced therapeutic effects on mice colitis, and PPAR-γ was found to be the main target of naringin by functional experiments both in vivo and in vitro. Our study supplies new scientific information for the use of naringin in colitis treatment. .
Keywords: NF-κB; RNA sequencing; inflammatory bowel disease; naringin; peroxisome proliferator-activated receptor-γ. V体育安卓版.
Copyright © 2021 Dong, Chen, Yang, Zhang, Wei, Xiong, Wang, Zhou, Li, Wang and Chen V体育ios版. .
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


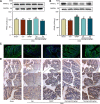
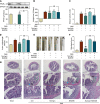

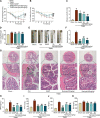

References
-
- Ahmad S. F., Attia S. M., Bakheet S. A., Zoheir K. M., Ansari M. A., Korashy H. M., et al. (2015). Naringin Attenuates the Development of Carrageenan-Induced Acute Lung Inflammation through Inhibition of NF-Κb, STAT3 and Pro-inflammatory Mediators and Enhancement of IκBα and Anti-inflammatory Cytokines. Inflammation 38, 846–857. 10.1007/s10753-014-9994-y - DOI - PubMed
-
- Ali M. M., El Kader M. A. (2004). The Influence of Naringin on the Oxidative State of Rats with Streptozotocin-Induced Acute Hyperglycaemia. Z. Naturforsch C J. Biosci. 59, 726–733. 10.1515/znc-2004-9-1018 - DOI (V体育平台登录) - PubMed
-
- Bassaganya-Riera J., Reynolds K., Martino-Catt S., Cui Y., Hennighausen L., Gonzalez F., et al. (2004). Activation of PPAR Gamma and delta by Conjugated Linoleic Acid Mediates protection from Experimental Inflammatory Bowel Disease. Gastroenterology 127, 777–791. 10.1053/j.gastro.2004.06.049 - DOI - PubMed
-
- Bolger A. M., Lohse M., Usadel B. (2014). Trimmomatic: a Flexible Trimmer for Illumina Sequence Data. Bioinformatics 30, 2114–2120. 10.1093/bioinformatics/btu170 - "V体育平台登录" DOI - PMC - PubMed
LinkOut - more resources
VSports注册入口 - Full Text Sources

