Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease
- PMID: 34494943
- PMCID: PMC8437544
- DOI: 10.1080/19490976.2021.1968257
Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease
Abstract
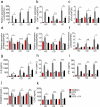
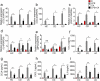
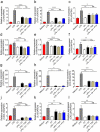
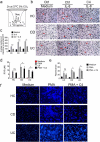
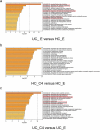
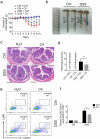
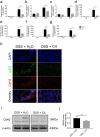
Host-microbial cross-talk plays a crucial role in maintenance of gut homeostasis. However, how microbiota-derived metabolites, e. g. , butyrate, regulate functions of neutrophils in the pathogenesis of inflammatory bowel disease (IBD) remains elusive. We sought to investigate the effects of butyrate on IBD neutrophils and elucidate the therapeutic potential in regulating mucosal inflammation. Peripheral neutrophils were isolated from IBD patients and healthy donors, and profiles of proinflammatory cytokines and chemokines were determined by qRT-PCR and ELISA, respectively. The migration and release of neutrophil extracellular traps (NETs) were studied by a Transwell model and immunofluorescence, respectively. The in vivo role of butyrate in regulating IBD neutrophils was evaluated in a DSS-induced colitis model in mice. We found that butyrate significantly inhibited IBD neutrophils to produce proinflammatory cytokines, chemokines, and calprotectins. Blockade of GPCR signaling with pertussis toxin (PTX) did not interfere the effects whereas pan-histone deacetylase (HDAC) inhibitor, trichostatin A (TSA) effectively mimicked the role of butyrate. Furthermore, in vitro studies confirmed that butyrate suppressed neutrophil migration and formation of NETs from both CD and UC patients. RNA sequencing analysis revealed that the immunomodulatory effects of butyrate on IBD neutrophils were involved in leukocyte activation, regulation of innate immune response and response to oxidative stress. Consistently, oral administration of butyrate markedly ameliorated mucosal inflammation in DSS-induced murine colitis through inhibition of neutrophil-associated immune responses such as proinflammatory mediators and NET formation. Our data thus reveal that butyrate constrains neutrophil functions and may serve as a novel therapeutic potential in the treatment of IBD VSports手机版. .
Keywords: Inflammatory bowel disease; butyrate; inflammatory mediators; neutrophil extracellular traps; neutrophils V体育安卓版. .
V体育2025版 - Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures (VSports注册入口)
References (V体育ios版)
-
- Rodríguez C, Romero E, Garrido-Sanchez L, Alcaín-Martínez G, Andrade RJ, Taminiau B, Daube G, García-Fuentes E. microbiota insights in clostridium difficile infection and inflammatory bowel disease. Gut Microbes. 2020;12(1):1725220. doi:10.1080/19490976.2020.1725220. - VSports最新版本 - DOI - PMC - PubMed
-
- Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson K, Glover L, Kominsky D, Magnuson A, et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids And Intestinal Epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17(5):662–671. doi:10.1016/j.chom.2015.03.005. - "V体育平台登录" DOI - PMC - PubMed
V体育官网入口 - Publication types
- "VSports手机版" Actions
MeSH terms
- "V体育2025版" Actions
- "V体育2025版" Actions
- "V体育平台登录" Actions
- "VSports app下载" Actions
- V体育官网入口 - Actions
- "V体育2025版" Actions
- "V体育官网入口" Actions
- "V体育ios版" Actions
- Actions (VSports app下载)
- VSports注册入口 - Actions
- "V体育平台登录" Actions
- VSports手机版 - Actions
- Actions (VSports在线直播)
- "V体育官网" Actions
- "VSports" Actions
Substances
- "V体育官网" Actions
- VSports - Actions
- "VSports在线直播" Actions
LinkOut - more resources
Full Text Sources (V体育平台登录)
"VSports" Research Materials
