V体育ios版 - Spatiotemporal and Functional Heterogeneity of Hematopoietic Stem Cell-Competent Hemogenic Endothelial Cells in Mouse Embryos
- PMID: 34458261
- PMCID: PMC8385538
- DOI: 10.3389/fcell.2021.699263
Spatiotemporal and Functional Heterogeneity of Hematopoietic Stem Cell-Competent Hemogenic Endothelial Cells in Mouse Embryos
Abstract
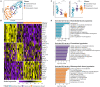
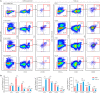
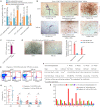
Hematopoietic stem cells (HSCs) are derived from hemogenic endothelial cells (HECs) during embryogenesis. The HSC-primed HECs increased to the peak at embryonic day (E) 10 and have been efficiently captured by the marker combination CD41-CD43-CD45-CD31+CD201+Kit+CD44+ (PK44) in the aorta-gonad-mesonephros (AGM) region of mouse embryos most recently. In the present study, we investigated the spatiotemporal and functional heterogeneity of PK44 cells around the time of emergence of HSCs. First, PK44 cells in the E10. 0 AGM region could be further divided into three molecularly different populations showing endothelial- or hematopoietic-biased characteristics. Specifically, with the combination of Kit, the expression of CD93 or CD146 could divide PK44 cells into endothelial- and hematopoietic-feature biased populations, which was further functionally validated at the single-cell level. Next, the PK44 population could also be detected in the yolk sac, showing similar developmental dynamics and functional diversification with those in the AGM region. Importantly, PK44 cells in the yolk sac demonstrated an unambiguous multilineage reconstitution capacity after in vitro incubation VSports手机版. Regardless of the functional similarity, PK44 cells in the yolk sac displayed transcriptional features different from those in the AGM region. Taken together, our work delineates the spatiotemporal characteristics of HECs represented by PK44 and reveals a previously unknown HSC competence of HECs in the yolk sac. These findings provide a fundamental basis for in-depth study of the different origins and molecular programs of HSC generation in the future. .
Keywords: developmental hematopoiesis; hematopoietic stem cells; hemogenic endothelial cells; heterogeneity; single-cell RNA-sequencing V体育安卓版. .
Copyright © 2021 Li, Gong, Hou, Huang, Wang, Liu, Ni, Wang, Wang, Hou, Yang, Yan, Zhang, Liu and Lan V体育ios版. .
V体育2025版 - Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Anders S., Pyl P. T., Huber W. (2015). HTSeq–a python framework to work with high-throughput sequencing data. Bioinformatics 31 166–169. 10.1093/bioinformatics/btu638 - DOI (V体育平台登录) - PMC - PubMed
-
- Chen M. J., Yokomizo T., Zeigler B. M., Dzierzak E., Speck N. A. (2009). Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457 887–891. 10.1038/nature07619 - "V体育安卓版" DOI - PMC - PubMed
LinkOut - more resources
"V体育官网" Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

