G protein coupled estrogen receptor attenuates mechanical stress-mediated apoptosis of chondrocyte in osteoarthritis via suppression of Piezo1
- PMID: 34454425
- PMCID: PMC8403401
- DOI: 10.1186/s10020-021-00360-w
G protein coupled estrogen receptor attenuates mechanical stress-mediated apoptosis of chondrocyte in osteoarthritis via suppression of Piezo1
Abstract (V体育官网入口)
Background: Apoptosis of chondrocyte is involved in osteoarthritis (OA) pathogenesis, and mechanical stress plays a key role in this process by activation of Piezo1. However, the negative regulation of signal conduction mediated by mechanical stress is still unclear. Here, we elucidate that the critical role of G protein coupled estrogen receptor (GPER) in the regulation of mechanical stress-mediated signal transduction and chondrocyte apoptosis. VSports手机版.
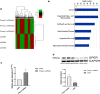
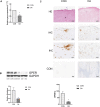
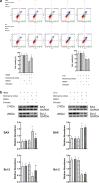
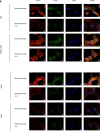
Methods: The gene expression profile was detected by gene chip upon silencing Piezo1. The expression of GPER in cartilage tissue taken from the clinical patients was detected by RT-PCR and Western blot as well as immunohistochemistry, and the correlation between GPER expression and OA was also investigated. The chondrocytes exposed to mechanical stress were treated with estrogen, G-1, G15, GPER-siRNA and YAP (Yes-associated protein)-siRNA. The cell viability of chondrocytes was measured. The expression of polymerized actin and Piezo1 as well as the subcellular localization of YAP was observed under laser confocal microscope. Western blot confirmed the changes of YAP/ Rho GTPase activating protein 29 (ARHGAP29) /RhoA/LIMK /Cofilin pathway. The knee specimens of osteoarthritis model were stained with safranin and green. OARSI score was used to evaluate the joint lesions. The expressions of GPER and YAP were detected by immunochemistry. V体育安卓版.
Results: Expression profiles of Piezo1- silenced chondrocytes showed that GPER expression was significantly upregulated. Moreover, GPER was negatively correlated with cartilage degeneration during OA pathogenesis. In addition, we uncovered that GPER directly targeted YAP and broadly restrained mechanical stress-triggered actin polymerization. Mechanism studies revealed that GPER inhibited mechanical stress-mediated RhoA/LIMK/cofilin pathway, as well as the actin polymerization, by promoting expression of YAP and ARHGAP29, and the YAP nuclear localization, eventually causing the inhibition of Piezo1. YAP was obviously decreased in degenerated cartilage. Silencing YAP caused significantly increased actin polymerization and activation of Piezo1, and an increase of chondrocyte apoptosis. In addition, intra-articular injection of G-1 to OA rat effectively attenuated cartilage degeneration. V体育ios版.
Conclusion: We propose a novel regulatory mechanism underlying mechanical stress-mediated apoptosis of chondrocyte and elucidate the potential application value of GPER as therapy targets for OA VSports最新版本. .
Keywords: Chondrocyte; G protein coupled estrogen receptor; Mechanical stress; Osteoarthritis; Piezo1 V体育平台登录. .
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests
Figures






References
-
- Alexander A, Irving AJ, Harvey J. Emerging roles for the novel estrogen-sensing receptor GPER1 in the CNS. Neuropharmacology. 2017;113:652–660. doi: 10.1016/j.neuropharm.2016.07.003. - "VSports注册入口" DOI - PubMed
Publication types
- "V体育ios版" Actions
MeSH terms
- Actions (VSports在线直播)
- Actions (V体育平台登录)
- Actions (V体育官网)
- "V体育ios版" Actions
- V体育2025版 - Actions
- "V体育ios版" Actions
- VSports注册入口 - Actions
- Actions (VSports app下载)
- V体育ios版 - Actions
- "V体育平台登录" Actions
- Actions (V体育官网)
- VSports最新版本 - Actions
Substances
- V体育官网入口 - Actions
- "VSports注册入口" Actions
- "V体育官网入口" Actions
- "V体育平台登录" Actions
- "VSports" Actions
- Actions (VSports手机版)
- Actions (VSports最新版本)
LinkOut - more resources
Full Text Sources
Medical (V体育ios版)

