Human Amniotic Mesenchymal Stem Cells Inhibit aGVHD by Regulating Balance of Treg and T Effector Cells (V体育官网入口)
- PMID: 34429630
- PMCID: PMC8378934 (VSports)
- DOI: 10.2147/JIR.S323054
VSports注册入口 - Human Amniotic Mesenchymal Stem Cells Inhibit aGVHD by Regulating Balance of Treg and T Effector Cells
Abstract
Background: Acute graft versus host disease (aGVHD) remains a leading cause of transplant-related mortality following allogeneic haematopoietic cell transplantation (allo-HCT). Human amniotic mesenchymal stem cells (hAMSCs) are a novel mesenchymal stem cells (MSCs), which have stronger proliferation and immunomodulatory ability compared with bone marrow mesenchymal stem cells (BM-MSCs) VSports手机版. Besides, as the amniotic membrane is often treated as medical waste after delivery, hAMSCs can be obtained conveniently and noninvasively. The aim of this study was to explore the therapeutic efficacy and underlying mechanisms of hAMSCs transplantation for the humanized aGVHD mouse model. .
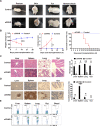
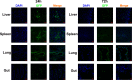
Methods: We established a humanized aGVHD mouse model by transplanting human peripheral blood mononuclear cells (PBMCs) into NOD-PrkdcscidIL2rγnull (NPG) mice, human amniotic membrane collected from discarded placenta of healthy pregnant women after delivery and hAMSCs were extracted from amniotic membrane and expanded in vitro. Mice were divided into untreated group (Control), aGVHD group (aGVHD), and hAMSCs treatment group (aGVHD+hAMSCs), the hAMSCs labeled with GFP were administered to aGVHD mice to explore the homing ability of hAMSCs. T effector and regulatory T cells (Tregs) levels and cytokines of each group in target organs were detected by flow cytometry and cytometric bead array (CBA), respectively V体育安卓版. .
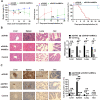
Results: We successfully established a humanized aGVHD mouse model using NPG mice. The hAMSCs have the ability to inhibit aGVHD in this mouse model through reduced villous blunting and lymphocyte infiltration of the gut while reducing inflammatory edema, tissue destruction and lymphocyte infiltration into the parenchyma of the liver and lung. hAMSCs suppressed CD3+CD4+ T and CD3+CD8+ T cell expression and increased the proportion of Tregs, and besides, hAMSCs can reduce the levels of IL-17A, INF-γ, and TNF in aGVHD target organs V体育ios版. .
Conclusion: The NPG murine environment was capable of activating human T cells to produce aGVHD pathology to mimic aGVHD as in humans. The hAMSCs controlled aGVHD by decreasing inflammatory cytokine secretion within target organs by modulating the balance of Tregs and T effector cells in humanized mice. VSports最新版本.
Keywords: NPG mice; acute graft versus host disease; amniotic mesenchymal stem cells; humanized mouse model; immunomodulatory. V体育平台登录.
© 2021 Gao et al.
Conflict of interest statement (VSports注册入口)
The authors report no conflicts of interest in this work.
Figures (VSports)


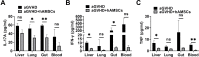
References
-
- Wolf D, von Lilienfeld-toal M, Wolf AM, et al. Novel treatment concepts for graft-versus-host disease. Blood. 2012;119(1):16–25. doi:10.1182/blood-2011-08-339465 - "V体育平台登录" DOI - PubMed
-
- Wu X, Jiang J, Gu Z, Zhang J, Chen Y, Liu X. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Res Ther. 2020;11(1):345. doi:10.1186/s13287-020-01855-9 - DOI (V体育2025版) - PMC - PubMed
LinkOut - more resources (VSports最新版本)
Full Text Sources
V体育官网入口 - Research Materials

