"V体育官网" Rationale and study design of a randomized, placebo-controlled, double-blind phase 2b trial to evaluate efficacy, safety, and tolerability of an oral glutaminyl cyclase inhibitor varoglutamstat (PQ912) in study participants with MCI and mild AD-VIVIAD
- PMID: 34425883
- PMCID: PMC8381483 (V体育安卓版)
- DOI: 10.1186/s13195-021-00882-9
Rationale and study design of a randomized, placebo-controlled, double-blind phase 2b trial to evaluate efficacy, safety, and tolerability of an oral glutaminyl cyclase inhibitor varoglutamstat (PQ912) in study participants with MCI and mild AD-VIVIAD (VSports app下载)
Abstract (V体育ios版)
Background: Varoglutamstat (formerly PQ912) is a small molecule that inhibits the activity of the glutaminyl cyclase to reduce the level of pyroglutamate-A-beta (pGluAB42). Recent studies confirm that pGluAB42 is a particular amyloid form that is highly synaptotoxic and plays a significant role in the development of AD VSports手机版. .
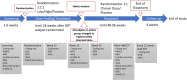
Methods: This paper describes the design and methodology behind the phase 2b VIVIAD-trial in AD V体育安卓版. The aim of this study is to evaluate varoglutamstat in a state-of-the-art designed, placebo-controlled, double-blind, randomized clinical trial for safety and tolerability, efficacy on cognition, and effects on brain activity and AD biomarkers. In addition to its main purpose, the trial will explore potential associations between novel and established biomarkers and their individual and composite relation to disease characteristics. .
Results: To be expected early 2023 CONCLUSION: This state of the art phase 2b study will yield important results for the field with respect to trial methodology and for the treatment of AD with a small molecule directed against pyroglutamate-A-beta V体育ios版. .
Trial registration: ClinicalTrials. gov Identifier: NCT04498650. VSports最新版本.
Keywords: Abeta; Alzheimer; Amyloid; Clinical trials; Small molecules; Puroglutamate; Placebo. V体育平台登录.
© 2021. The Author(s).
VSports app下载 - Conflict of interest statement
Dr. Scheltens has received consultancy fees (paid to the institution) from AC Immune, Alkermes, Alnylam, Alzheon, Anavex, Biogen, Brainstorm Cell, Cortexyme, Denali, EIP, ImmunoBrain Checkpoint, GemVax, Genentech, Green Valley, Novartis, Novo Nordisk, PeopleBio, Renew LLC, and Roche VSports注册入口. He is PI of studies with AC Immune, CogRx, FUJI-film/Toyama, IONIS, UCB, and Vivoryon. He is a part-time employee of Life Sciences Partners Amsterdam. He serves on the board of the Brain Research Center.
Dr. Vijverberg has received consultancy fees (paid to the institution) from Biogen, Brainstorm Cell, ImmunoBrain Checkpoint, New Amsterdam Pharma, and Treeway. He is PI of studies with AC Immune, CogRx, Green Valley, IONIS, Janssen, Roche, Rodin Therapeutics, Sanofi, UCB, and Vivoryon V体育官网入口.
Dr VSports在线直播. Harrison has received consultancy fees from 23andMe, Alkahest, AlzeCure, Aptinyx, Athira Pharma, Axon, Axovant, Bial, Biogen, BlackthornRx, Boehringer Ingelheim, Brands2Life, Cognition Therapeutics, Compass Pathways, Corlieve, Cronos, Curasen, DeNDRoN, Eisai, EIP Pharma, Eli Lilly, FSV7, G4X Discovery, GfHEU, Heptares, Imperial College, Johnson & Johnson, Kaasa Health, Ki-Elements, Lundbeck, Lysosome Therapeutics, Merck, Neurocentria, Neurocog, Neurodyn, Neurotrack, NHS, Novartis, Nutricia, Probiodrug, Regeneron, reMYND, Rodin Therapeutics, Roivant, Samumed, Sanofi, Signant Health, Syndesi Therapeutics, Takeda, Virusgenics, Vivoryon, and WinterLight Labs.
Dr. Axelsen has no conflicts of interest to declare.
Dr. Weber is a part-time employee of Vivoryon Therapeutics NV, Germany.
AR. Bihlet is a full-time employee of NBCD A/S, a clinical contract-research organization.
K. Kuehn-Wache and K. Fuchs are a full-time employees of Vivoryon Therapeutics NV, Germany.
Dr. Prins is consultant to Boehringer Ingelheim and Aribio. He is co-PI of studies with EIP Pharma and Fuji Film Toyama Chemical. He serves on the DSMB of Abbvie’s M15-566 trial. He is CEO and co-owner of the Brain Research Center, The Netherlands.
Figures
References
-
- Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer’s disease drug development pipeline: 2020. Alzheimers Dement. 2020. - "V体育安卓版" PMC - PubMed
-
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95(11):6448–6453. doi: 10.1073/pnas.95.11.6448. - DOI (VSports手机版) - PMC - PubMed
-
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. - "VSports在线直播" DOI - PubMed
-
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14(8):837–842. doi: 10.1038/nm1782. - DOI - PMC - PubMed
Publication types
- "VSports在线直播" Actions
VSports - MeSH terms
- Actions (VSports app下载)
- "V体育平台登录" Actions
Substances
"V体育安卓版" Associated data
- "V体育2025版" Actions
LinkOut - more resources
Full Text Sources
Medical

