"V体育官网" Gasdermin D inhibition prevents multiple organ dysfunction during sepsis by blocking NET formation
- PMID: 34407544
- PMCID: PMC8703366
- DOI: 10.1182/blood.2021011525 (VSports)
VSports注册入口 - Gasdermin D inhibition prevents multiple organ dysfunction during sepsis by blocking NET formation
Abstract
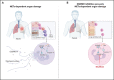
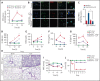
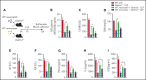
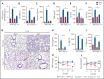
Multiple organ dysfunction is the most severe outcome of sepsis progression and is highly correlated with a worse prognosis. Excessive neutrophil extracellular traps (NETs) are critical players in the development of organ failure during sepsis. Therefore, interventions targeting NET release would likely effectively prevent NET-based organ injury associated with this disease. Herein, we demonstrate that the pore-forming protein gasdermin D (GSDMD) is active in neutrophils from septic humans and mice and plays a crucial role in NET release. Inhibition of GSDMD with disulfiram or genic deletion abrogated NET formation, reducing multiple organ dysfunction and sepsis lethality VSports手机版. Mechanistically, we demonstrate that during sepsis, activation of the caspase-11/GSDMD pathway controls NET release by neutrophils during sepsis. In summary, our findings uncover a novel therapeutic use for disulfiram and suggest that GSDMD is a therapeutic target to improve sepsis treatment. .
© 2021 by The American Society of Hematology. V体育安卓版.
Figures


Comment in
-
Old drug revisited: disulfiram, NETs, and sepsis.Blood. 2021 Dec 23;138(25):2604-2605. doi: 10.1182/blood.2021013438. Blood. 2021. PMID: 34940816 No abstract available.
References
-
- Singer M, Deutschman CS, Seymour CW, et al. . The third international consensus definitions for 492 sepsis and septic shock (Sepsis-3). JAMA 2016;315:801-810. - PMC (V体育安卓版) - PubMed
-
- Brown KA, Brain SD, Pearson JD, Edgeworth JD, Lewis SM, Treacher DF. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368(9530):157-169. - VSports app下载 - PubMed
-
- Pool R, Gomez H, Kellum JA. Mechanisms of organ dysfunction in sepsis. Crit Care Clin. 2018;34(1):63-80. - V体育平台登录 - PMC - PubMed
-
- Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159-175. - PubMed
-
- Kruger P, Saffarzadeh M, Weber AN, et al. . Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11(3):e1004651. - "V体育官网入口" PMC - PubMed
Publication types
MeSH terms
- "V体育官网" Actions
- Actions (V体育2025版)
- V体育2025版 - Actions
- "V体育平台登录" Actions
- VSports - Actions
- VSports注册入口 - Actions
- Actions (VSports手机版)
- V体育官网入口 - Actions
- VSports手机版 - Actions
- Actions (VSports在线直播)
Substances (VSports手机版)
- VSports最新版本 - Actions
- VSports最新版本 - Actions
- Actions (V体育官网入口)

