A phase Ib/IIa trial of 9 repurposed drugs combined with temozolomide for the treatment of recurrent glioblastoma: CUSP9v3
- PMID: 34377985
- PMCID: PMC8349180
- DOI: 10.1093/noajnl/vdab075
A phase Ib/IIa trial of 9 repurposed drugs combined with temozolomide for the treatment of recurrent glioblastoma: CUSP9v3
"V体育安卓版" Abstract
Background: The dismal prognosis of glioblastoma (GBM) may be related to the ability of GBM cells to develop mechanisms of treatment resistance. We designed a protocol called Coordinated Undermining of Survival Paths combining 9 repurposed non-oncological drugs with metronomic temozolomide-version 3-(CUSP9v3) to address this issue. The aim of this phase Ib/IIa trial was to assess the safety of CUSP9v3. VSports手机版.
Methods: Ten adults with histologically confirmed GBM and recurrent or progressive disease were included V体育安卓版. Treatment consisted of aprepitant, auranofin, celecoxib, captopril, disulfiram, itraconazole, minocycline, ritonavir, and sertraline added to metronomic low-dose temozolomide. Treatment was continued until toxicity or progression. Primary endpoint was dose-limiting toxicity defined as either any unmanageable grade 3-4 toxicity or inability to receive at least 7 of the 10 drugs at ≥ 50% of the per-protocol doses at the end of the second treatment cycle. .
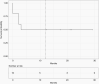
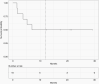
Results: One patient was not evaluable for the primary endpoint (safety). All 9 evaluable patients met the primary endpoint. Ritonavir, temozolomide, captopril, and itraconazole were the drugs most frequently requiring dose modification or pausing. The most common adverse events were nausea, headache, fatigue, diarrhea, and ataxia. Progression-free survival at 12 months was 50%. V体育ios版.
Conclusions: CUSP9v3 can be safely administered in patients with recurrent GBM under careful monitoring. A randomized phase II trial is in preparation to assess the efficacy of the CUSP9v3 regimen in GBM. VSports最新版本.
Keywords: chemotherapy; clinical trial; drug repurposing; glioblastoma; multi-drug combination. V体育平台登录.
© The Author(s) 2021. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology VSports注册入口. .
Figures
References
-
- Lakomy R, Kazda T, Selingerova I, et al. . Real-world evidence in glioblastoma: stupp’s regimen after a decade. Front Oncol. 2020;10:840. - "V体育平台登录" PMC - PubMed
-
- Mandel JJ, Yust-Katz S, Patel AJ, et al. . Inability of positive phase II clinical trials of investigational treatments to subsequently predict positive phase III clinical trials in glioblastoma. Neuro Oncol. 2018;20(1):113–122. - "VSports最新版本" PMC - PubMed
-
- Kast RE, Boockvar JA, Brüning A, et al. . A conceptually new treatment approach for relapsed glioblastoma: coordinated undermining of survival paths with nine repurposed drugs (CUSP9) by the International Initiative for Accelerated Improvement of Glioblastoma Care. Oncotarget. 2013;4(4):502–530. - PMC - PubMed
-
- Serafin MB, Bottega A, da Rosa TF, et al. . Drug repositioning in oncology. Am J Ther. 2021;28(1):e111–e117. - PubMed
LinkOut - more resources
Full Text Sources
V体育2025版 - Miscellaneous