Levobupivacaine Induces Ferroptosis by miR-489-3p/SLC7A11 Signaling in Gastric Cancer
- PMID: 34177591
- PMCID: PMC8220201
- DOI: 10.3389/fphar.2021.681338
Levobupivacaine Induces Ferroptosis by miR-489-3p/SLC7A11 Signaling in Gastric Cancer
Abstract
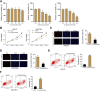
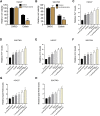
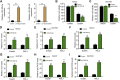
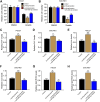
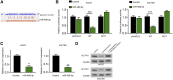
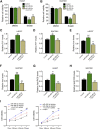
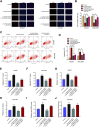
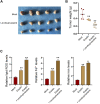
Gastric cancer is one of the most the prevalent malignancies and the therapeutic strategies for patients with gastric cancer remains limited. Local anesthetic levobupivacaine has demonstrated potential anti-cancer property, but its correlation with gastric cancer and ferroptosis is poor understood. Here, we identified the novel function of levobupivacaine in regulating ferroptosis of gastric cancer cells. The treatment of levobupivacaine suppressed gastric cancer cell viabilities and Edu-positive cell proportions. The gastric cancer cell growth was reduced by levobupivacaine in vivo. Moreover, the treatment of levobupivacaine enhanced erastin-induced inhibitory impact on gastric cancer cell viabilities. The levels of Fe2+/iron and lipid ROS were induced by levobupivacaine in erastin and RSL3-stimulated gastric cancer cells. levobupivacaine-upregulated miR-489-3p enhanced ferroptosis of gastric cancer cells by targeting SLC7A11 VSports手机版. MiR-489-3p was involved in levobupivacaine-induced ferroptosis of gastric cancer cells. Levobupivacaine/miR-489-3p/SLC7A11 axis attenuates gastric cancer cell proliferation in vitro. Therefore, we concluded that the local anesthetic levobupivacaine induced ferroptosis of gastric cancer cells to repress gastric cancer cell growth by miR-489-3p/SLC7A11 axis. .
Keywords: SLC7A11; ferroptosis; gastric cancer; levobupivacaine; mir-489-3p. V体育安卓版.
Copyright © 2021 Mao, Zhu, Nie, Yu and Wang V体育ios版. .
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Alvarez S. W., Sviderskiy V. O., Terzi E. M., Papagiannakopoulos T., Moreira A. L., Adams S., et al. (2017). NFS1 Undergoes Positive Selection in Lung Tumours and Protects Cells from Ferroptosis. Nature 551, 639–643. 10.1038/nature24637 - "VSports app下载" DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

