Upadacitinib Treatment Improves Symptoms of Bowel Urgency and Abdominal Pain, and Correlates With Quality of Life Improvements in Patients With Moderate to Severe Ulcerative Colitis
- PMID: 34107013
- PMCID: PMC8684481 (V体育官网)
- DOI: 10.1093/ecco-jcc/jjab099
Upadacitinib Treatment Improves Symptoms of Bowel Urgency and Abdominal Pain, and Correlates With Quality of Life Improvements in Patients With Moderate to Severe Ulcerative Colitis
V体育2025版 - Abstract
Background and aims: Bowel urgency and abdominal pain are impactful, yet under-appreciated ulcerative colitis symptoms and not commonly assessed in clinical trials. We evaluated how these symptoms may improve with upadacitinib treatment and correlate with clinical and health-related quality of life [HRQOL] outcomes in the phase 2b U-ACHIEVE study. VSports手机版.
Methods: Patients aged 18-75 years, with moderately to severely active ulcerative colitis, were randomised to receive placebo or upadacitinib (7. 5, 15, 30, or 45 mg once daily [QD]). Bowel urgency and abdominal pain were evaluated at baseline and Weeks 2, 4, 6, and 8. Week 8 correlations were evaluated between bowel urgency/abdominal pain with clinical [Mayo subscores and high-sensitivity C-reactive protein and faecal calprotectin measurements] and HRQOL outcomes [Inflammatory Bowel Disease Questionnaire and 36-Item Short Form Health Survey scores]. V体育安卓版.
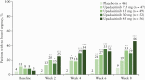
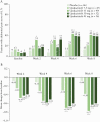
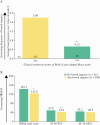
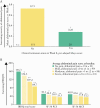
Results: A greater proportion of patients [n = 250] reported no bowel urgency and less abdominal pain with upadacitinib treatment compared with placebo, with improvements observed as early as 2 weeks. At Week 8, patients receiving the 45-mg QD dose had the greatest improvements versus placebo, with 46% reporting no bowel urgency [vs 9%; p ≤ 0. 001] and 38% reporting no abdominal pain [vs 13%; p = 0 V体育ios版. 015]. At Week 8, moderate correlations were found between bowel urgency or abdominal pain and most clinical and HRQOL outcomes. .
Conclusions: Induction treatment with upadacitinib demonstrated significant reductions in bowel urgency and abdominal pain compared with placebo. These symptoms also correlate to clinical and HRQOL outcomes, supporting their use to monitor disease severity and other treatment outcomes VSports最新版本. .
Keywords: Abdominal pain; bowel urgency; upadacitinib V体育平台登录. .
© The Author(s) 2021. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation. VSports注册入口.
Figures
VSports - References
-
- Ng SC, Shi HY, Hamidi N, et al. . Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 2017;390:2769–78. - PubMed
-
- National Institute for Health and Care Excellence. Ulcerative Colitis: Management. NICE guideline. 2019. https://www.nice.org.uk/guidance/ng130 Accessed July 1, 2019. - PubMed
-
- Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019;114:384–413. - PubMed
-
- Bilsborough J, Targan SR, Snapper SB. Therapeutic targets in inflammatory bowel disease: current and future. Am J Gastroenterol Suppl 2016;3:27–37.
Publication types
- VSports注册入口 - Actions
MeSH terms
- "VSports注册入口" Actions
- Actions (V体育ios版)
- "V体育官网入口" Actions
- "V体育2025版" Actions
- VSports最新版本 - Actions
- Actions (VSports在线直播)
- Actions (V体育2025版)
- VSports app下载 - Actions
- VSports - Actions
- Actions (V体育安卓版)
- Actions (V体育安卓版)
- V体育官网 - Actions
Substances
- Actions (VSports app下载)
- "V体育安卓版" Actions

