MiR-375 reduces the stemness of gastric cancer cells through triggering ferroptosis
- PMID: 34090492
- PMCID: PMC8180146
- DOI: 10.1186/s13287-021-02394-7 (V体育2025版)
MiR-375 reduces the stemness of gastric cancer cells through triggering ferroptosis
Abstract
Background: Gastric cancer stem cells (CSCs) are the main causes of metastasis and drug resistance. We previously indicated that miR-375 can inhibit Helicobacter pylori-induced gastric carcinogenesis; here, we aim to explore the effects and mechanisms of miR-375 on gastric cancer (GC) cell stemness VSports手机版. .
Methods: Lentivirus infection was used to construct GC cells with ectopic expression of miR-375. In vitro and in vivo experiments, including analysis of tumor spheroid formation, CD44+ sub-population with stemness, stemness marker expression, and tumor-initiating ability, were performed to evaluate the effects of miR-375 on the stemness of GC cells. Furthermore, microarray and bioinformatics analysis were performed to search the potential targets of miR-375 in GC cells. Luciferase reporter, RNA immunoprecipitation, and RNA-FISH assays were carried out to verify the targeting of miR-375. Subsequently, combined with tissue microarray analysis, erastin-resistant GC cells, transmission electron microscopy, a series of agonists and oxidative stress markers, the underlying mechanisms contributing to miR-375-mediated effects were explored V体育安卓版. .
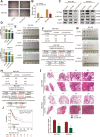
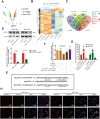
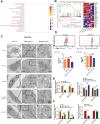
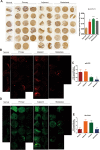
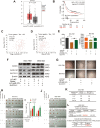
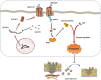
Results: MiR-375 reduced the stemness of GC cells in vitro and in vivo. Mechanistically, SLC7A11 was identified as a direct target of miR-375 and miR-375 attenuated the stemness of GC cells mainly through triggering SLC7A11-dependent ferroptosis V体育ios版. .
Conclusion: MiR-375 can trigger the ferroptosis through targeting SLC7A11, which is essential for miR-375-mediated inhibition on GC cell stemness. These results suggest that the miR-375/SLC7A11 regulatory axis could serve as a potential target to provoke the ferroptosis and thus attenuate the stemness of GC cells VSports最新版本. .
Keywords: Ferroptosis; Gastric cancer; MiR-375; SLC7A11; Stemness V体育平台登录. .
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, III, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042. - DOI (V体育ios版) - PMC - PubMed
Publication types
MeSH terms
- "VSports最新版本" Actions
- V体育2025版 - Actions
- V体育官网 - Actions
- "V体育官网入口" Actions
Substances
- Actions (V体育2025版)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

