A Cystine-Cysteine Intercellular Shuttle Prevents Ferroptosis in xCTKO Pancreatic Ductal Adenocarcinoma Cells
- PMID: 33801101
- PMCID: PMC8004104
- DOI: 10.3390/cancers13061434
A Cystine-Cysteine Intercellular Shuttle Prevents Ferroptosis in xCTKO Pancreatic Ductal Adenocarcinoma Cells
V体育官网入口 - Abstract
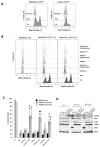
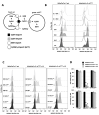
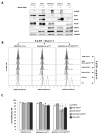
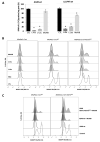
In our previous study, we showed that a cystine transporter (xCT) plays a pivotal role in ferroptosis of pancreatic ductal adenocarcinoma (PDAC) cells in vitro. However, in vivo xCTKO cells grew normally indicating that a mechanism exists to drastically suppress the ferroptotic phenotype. We hypothesized that plasma and neighboring cells within the tumor mass provide a source of cysteine to confer full ferroptosis resistance to xCTKO PDAC cells. To evaluate this hypothesis, we (co-) cultured xCTKO PDAC cells with different xCT-proficient cells or with their conditioned media. Our data unequivocally showed that the presence of a cysteine/cystine shuttle between neighboring cells is the mechanism that provides redox and nutrient balance, and thus ferroptotic resistance in xCTKO cells. Interestingly, although a glutathione shuttle between cells represents a good alternative hypothesis as a "rescue-mechanism", our data clearly demonstrated that the xCTKO phenotype is suppressed even with conditioned media from cells lacking the glutathione biosynthesis enzyme. Furthermore, we demonstrated that prevention of lipid hydroperoxide accumulation in vivo is mediated by import of cysteine into xCTKO cells via several genetically and pharmacologically identified transporters (ASCT1, ASCT2, LAT1, SNATs). Collectively, these data highlight the importance of the tumor environment in the ferroptosis sensitivity of cancer cells VSports手机版. .
Keywords: cysteine transporters; cysteine-cystine shuttle; ferroptosis; resistance; tumor environment V体育安卓版. .
Conflict of interest statement
The authors declare no conflict of interest.
V体育官网入口 - Figures

References
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. - DOI (VSports在线直播) - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

