A single strain of Bacteroides fragilis protects gut integrity and reduces GVHD
- PMID: 33554953
- PMCID: PMC7934839
- DOI: "V体育官网入口" 10.1172/jci.insight.136841
A single strain of Bacteroides fragilis protects gut integrity and reduces GVHD
Abstract
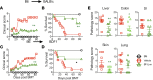
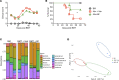
Graft-versus-host disease (GVHD) is a pathological process caused by an exaggerated donor lymphocyte response to host antigens after allogeneic hematopoietic cell transplantation (allo-HCT). Donor T cells undergo extensive clonal expansion and differentiation, which culminate in damage to recipient target organs. Damage to the gastrointestinal tract is a main contributor to morbidity and mortality. The loss of diversity among intestinal bacteria caused by pretransplant conditioning regimens leads to an outgrowth of opportunistic pathogens and exacerbated GVHD after allo-HCT. Using murine models of allo-HCT, we found that an increase of Bacteroides in the intestinal microbiota of the recipients was associated with reduced GVHD in mice given fecal microbial transplantation. Administration of Bacteroides fragilis through oral gavage increased gut microbiota diversity and beneficial commensal bacteria and significantly ameliorated acute and chronic GVHD development. Preservation of gut integrity following B. fragilis exposure was likely attributed to increased short chain fatty acids, IL-22, and regulatory T cells, which in turn improved gut tight junction integrity and reduced inflammatory cytokine production of pathogenic T cells. The current study provides a proof of concept that a single strain of commensal bacteria can be a safe and effective means to protect gut integrity and ameliorate GVHD after allo-HCT VSports手机版. .
Keywords: Adaptive immunity; Bone marrow transplantation; Cancer immunotherapy; Immunology; Transplantation V体育安卓版. .
Conflict of interest statement
Figures







References
-
- Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95(9):2754–2759. doi: 10.1182/blood.V95.9.2754.009k25_2754_2759. - V体育ios版 - DOI - PubMed
Publication types
"V体育官网" MeSH terms
- "VSports在线直播" Actions
- V体育官网 - Actions
- V体育平台登录 - Actions
- "V体育官网入口" Actions
- V体育平台登录 - Actions
- "V体育官网入口" Actions
- Actions (VSports app下载)
- "V体育安卓版" Actions
Substances
- "VSports注册入口" Actions
V体育2025版 - Grants and funding
LinkOut - more resources (VSports)
Full Text Sources
Other Literature Sources (V体育平台登录)

