Sepsis plasma-derived exosomal miR-1-3p induces endothelial cell dysfunction by targeting SERP1
- PMID: 33416075
- PMCID: V体育安卓版 - PMC7843403
- DOI: 10.1042/CS20200573
Sepsis plasma-derived exosomal miR-1-3p induces endothelial cell dysfunction by targeting SERP1
Abstract
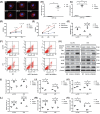
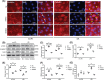
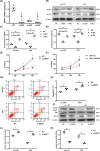
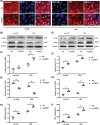
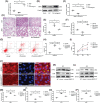
Acute lung injury (ALI) is the leading cause of death in sepsis patients. Exosomes participate in the occurrence and development of ALI by regulating endothelial cell inflammatory response, oxidative stress and apoptosis, causing serious pulmonary vascular leakage and interstitial edema. The current study investigated the effect of exosomal miRNAs on endothelial cells during sepsis. We found a significant increase in miR-1-3p expression in cecal ligation and puncture (CLP) rats exosomes sequencing and sepsis patients' exosomes, and lipopolysaccharide (LPS)-stimulated human umbilical vein endothelial cells (HUVECs) in vitro. However, the specific biological function of miR-1-3p in ALI remains unknown. Therefore, mimics or inhibitors of miR-1-3p were transfected to modulate its expression in HUVECs. Cell proliferation, apoptosis, contraction, permeability, and membrane injury were examined via cell counting kit-8 (CCK-8), flow cytometry, phalloidin staining, Transwell assay, lactate dehydrogenase (LDH) activity, and Western blotting. The miR-1-3p target gene was predicted with miRNA-related databases and validated by luciferase reporter. Target gene expression was blocked by siRNA to explore the underlying mechanisms. The results illustrated increased miR-1-3p and decreased stress-associated endoplasmic reticulum protein 1 (SERP1) expression both in vivo and in vitro. SERP1 was a direct target gene of miR-1-3p. Up-regulated miR-1-3p inhibits cell proliferation, promotes apoptosis and cytoskeleton contraction, increases monolayer endothelial cell permeability and membrane injury by targeting SERP1, which leads to dysfunction of endothelial cells and weakens vascular barrier function involved in the development of ALI. MiR-1-3p and SERP1 may be promising therapeutic candidates for sepsis-induced lung injury VSports手机版. .
Keywords: SERP1; endothelial cell dysfunction; exosomal miR-1-3p; sepsis-induced lung injury V体育安卓版. .
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
"V体育2025版" Figures


References
-
- Miyashita T., Ahmed A.K., Nakanuma S., Okamoto K., Sakai S., Kinoshita J. et al. . (2016) A three-phase approach for the early identification of acute lung injury induced by severe sepsis. In Vivo (Brooklyn) 30, 341–349, http://www.ncbi.nlm.nih.gov/pubmed/27381595 - PubMed
-
- Park E.J., Appiah M.G., Myint P.K., Gaowa A., Kawamoto E. and Shimaoka M. (2019) Exosomes in sepsis and inflammatory tissue injury. Curr. Pharm. Des. 25,4486–4495 - PubMed
Publication types
- V体育平台登录 - Actions
MeSH terms (VSports最新版本)
- "V体育安卓版" Actions
- "V体育官网" Actions
- "V体育官网入口" Actions
- Actions (VSports手机版)
- "VSports最新版本" Actions
- Actions (V体育2025版)
- V体育2025版 - Actions
- "V体育ios版" Actions
- "VSports在线直播" Actions
- "V体育官网" Actions
- VSports在线直播 - Actions
- Actions (V体育官网入口)
- Actions (V体育官网)
"VSports注册入口" Substances
- Actions (VSports最新版本)
- "VSports注册入口" Actions
- Actions (VSports在线直播)
LinkOut - more resources
VSports注册入口 - Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

