E3 ligase FBXW7 restricts M2-like tumor-associated macrophage polarization by targeting c-Myc
- PMID: 33260160
- PMCID: PMC7762499
- DOI: 10.18632/aging.202293
E3 ligase FBXW7 restricts M2-like tumor-associated macrophage polarization by targeting c-Myc (V体育安卓版)
Abstract
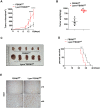
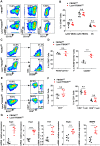
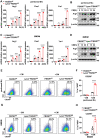
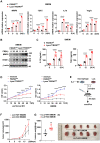
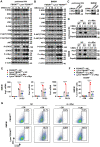
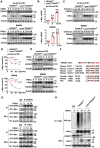
FBXW7 functions as an E3 ubiquitin ligase to mediate oncoprotein degradation via the ubiquitin-proteasome system in cancer cells, effectively inhibiting the growth and survival of tumor cells. However, little is known about the functions of FBXW7 in macrophages and the tumor immune microenvironment. In this study, we find that FBXW7 suppresses M2-like tumor-associated macrophage (TAM) polarization to limit tumor progression. We identified a significant increase in the proportion of M2-like TAMs and aggravated tumor growth in mice with myeloid FBXW7 deficiency by subcutaneous inoculation with Lewis lung carcinoma cells (LLCs). When stimulated with LLCs supernatant in vitro, FBXW7-knockout macrophages displayed increased M2 macrophage polarization and enhanced ability of supporting cancer cells growth. In mechanism, we confirmed that FBXW7 inhibited M2-like TAM polarization by mediating c-Myc degradation via the ubiquitin-proteasome system VSports手机版. These findings highlight the role of FBXW7 in M2-like TAM polarization and provide new insights into the potential targets for cancer immunotherapies. .
Keywords: FBXW7; c-Myc; macrophage polarization; tumor-associated macrophages; ubiquitination. V体育安卓版.
Conflict of interest statement
"VSports" Figures

References
-
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010; 11:889–96. 10.1038/ni.1937 - DOI (V体育官网入口) - PubMed
-
- Di Caro G, Cortese N, Castino GF, Grizzi F, Gavazzi F, Ridolfi C, Capretti G, Mineri R, Todoric J, Zerbi A, Allavena P, Mantovani A, Marchesi F. Dual prognostic significance of tumour-associated macrophages in human pancreatic adenocarcinoma treated or untreated with chemotherapy. Gut. 2016; 65:1710–20. 10.1136/gutjnl-2015-309193 - DOI - PubMed
-
- Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014; 513:559–63. 10.1038/nature13490 - "V体育官网" DOI - PMC - PubMed
-
- Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JA, Tamagnone L, Mazzone M. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013; 24:695–709. 10.1016/j.ccr.2013.11.007 - DOI - PubMed
Publication types
V体育官网入口 - MeSH terms
- "VSports注册入口" Actions
- VSports手机版 - Actions
- V体育平台登录 - Actions
- VSports最新版本 - Actions
- "VSports注册入口" Actions
- Actions (VSports)
- "VSports" Actions
- V体育2025版 - Actions
- VSports app下载 - Actions
Substances
- "V体育官网入口" Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

