Discovery of a novel ferroptosis inducer-talaroconvolutin A-killing colorectal cancer cells in vitro and in vivo
- PMID: 33203867
- PMCID: PMC7673992
- DOI: VSports - 10.1038/s41419-020-03194-2
Discovery of a novel ferroptosis inducer-talaroconvolutin A-killing colorectal cancer cells in vitro and in vivo
Abstract
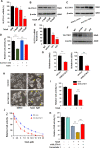
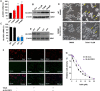
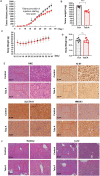
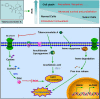
Ferropotsis is among the most important mechanisms of cancer suppression, which could be harnessed for cancer therapy. However, no natural small-molecule compounds with cancer inhibitory activity have been identified to date VSports手机版. In the present study, we reported the discovery of a novel ferroptosis inducer, talaroconvolutin A (TalaA), and the underlying molecular mechanism. We discovered that TalaA killed colorectal cancer cells in dose-dependent and time-dependent manners. Interestingly, TalaA did not induce apoptosis, but strongly triggered ferroptosis. Notably, TalaA was significantly more effective than erastin (a well-known ferroptosis inducer) in suppressing colorectal cancer cells via ferroptosis. We revealed a dual mechanism of TalaA' action against cancer. On the one hand, TalaA considerably increased reactive oxygen species levels to a certain threshold, the exceeding of which induced ferroptosis. On the other hand, this compound downregulated the expression of the channel protein solute carrier family 7 member 11 (SLC7A11) but upregulated arachidonate lipoxygenase 3 (ALOXE3), promoting ferroptosis. Furthermore, in vivo experiments in mice evidenced that TalaA effectively suppressed the growth of xenografted colorectal cancer cells without obvious liver and kidney toxicities. The findings of this study indicated that TalaA could be a new potential powerful drug candidate for colorectal cancer therapy due to its outstanding ability to kill colorectal cancer cells via ferroptosis induction. .
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
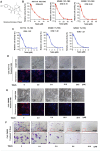
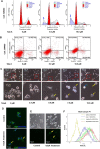
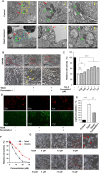
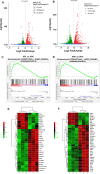
References
-
- Levine O, Zbuk K. Colorectal cancer in adolescents and young adults: defining a growing threat. Pediatr. Blood Cancer. 2019;66:e27941. doi: 10.1002/pbc.27941. - V体育官网 - DOI - PubMed
Publication types
- Actions (V体育安卓版)
MeSH terms
- "V体育平台登录" Actions
- "V体育ios版" Actions
- VSports app下载 - Actions
- VSports在线直播 - Actions
- Actions (V体育安卓版)
- Actions (V体育安卓版)
"VSports手机版" Substances
- "VSports手机版" Actions
- VSports在线直播 - Actions
LinkOut - more resources
Full Text Sources
Medical

