"V体育2025版" Gut Microbiota and Immune System Interactions
- PMID: 33076307
- PMCID: PMC7602490 (VSports最新版本)
- DOI: 10.3390/microorganisms8101587
Gut Microbiota and Immune System Interactions
Erratum in
-
Correction: Yoo, J.Y., et al. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587.Microorganisms. 2020 Dec 21;8(12):2046. doi: 10.3390/microorganisms8122046. Microorganisms. 2020. PMID: 33371530 Free PMC article.
Abstract
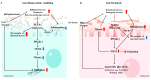
Dynamic interactions between gut microbiota and a host's innate and adaptive immune systems play key roles in maintaining intestinal homeostasis and inhibiting inflammation VSports手机版. The gut microbiota metabolizes proteins and complex carbohydrates, synthesize vitamins, and produce an enormous number of metabolic products that can mediate cross-talk between gut epithelial and immune cells. As a defense mechanism, gut epithelial cells produce a mucosal barrier to segregate microbiota from host immune cells and reduce intestinal permeability. An impaired interaction between gut microbiota and the mucosal immune system can lead to an increased abundance of potentially pathogenic gram-negative bacteria and their associated metabolic changes, disrupting the epithelial barrier and increasing susceptibility to infections. Gut dysbiosis, or negative alterations in gut microbial composition, can also dysregulate immune responses, causing inflammation, oxidative stress, and insulin resistance. Over time, chronic dysbiosis and the translocation of bacteria and their metabolic products across the mucosal barrier may increase prevalence of type 2 diabetes, cardiovascular disease, inflammatory bowel disease, autoimmune disease, and a variety of cancers. In this paper, we highlight the pivotal role gut microbiota and their metabolites (short-chain fatty acids (SCFAs)) play in mucosal immunity. .
Keywords: gut dysbiosis; gut microbiota; gut microbiota metabolites; immune system; short-chain fatty acids (SCFAs). V体育安卓版.
Conflict of interest statement
The authors declare no conflict of interest.
"V体育2025版" Figures


References
-
- Belkaid Y., Hand T.W. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. - VSports app下载 - DOI - PMC - PubMed
-
- Venegas D.P., Marjorie K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J., Faber K.N., Hermoso M.A. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019;43:629–631. doi: 10.1016/j.immuni.2015.09.014. - DOI - PMC - PubMed
-
- Macfarlane G., Gibson G. Physiological and Clinical Aspects of Short-Chain Fatty Acids. Cambridge University Press; Cambridge, UK: 1995. Microbiological aspects of the production of short-chain fatty acids in the large bowel; pp. 87–105.
Publication types
- "V体育2025版" Actions
"VSports app下载" LinkOut - more resources
"V体育官网入口" Full Text Sources
Research Materials

