Formononetin Attenuates Airway Inflammation and Oxidative Stress in Murine Allergic Asthma
- PMID: 33013383
- PMCID: PMC7500463
- DOI: 10.3389/fphar.2020.533841
Formononetin Attenuates Airway Inflammation and Oxidative Stress in Murine Allergic Asthma
Abstract
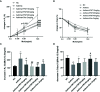
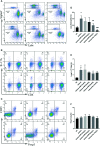
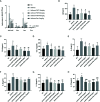
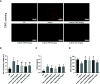
Allergic asthma has been considered as a respiratory disorder with pathological features of airway inflammation and remodeling, which involves oxidative stress. Formononetin (FMT) is a bioactive isoflavone obtained from Chinese herb Radix Astragali, and has been reported to have notable anti-inflammatory and antioxidant effects in several diseases. The purpose of our study was to elaborate the effects of FMT on asthma and the underlying mechanisms. To establish allergic asthma model, BALB/c mice were given ovalbumin (OVA) sensitization and challenge, treated with FMT (10, 20, 40 mg/kg) or dexamethasone (2 mg/kg). The effects of FMT on lung inflammation and oxidative stress were assessed. In OVA-induced asthmatic mice, FMT treatments significantly ameliorated lung function, alleviated lung inflammation including infiltration of inflammatory cells, the elevated levels of interleukin (IL)-4, IL-5, and IL-13, immunoglobulin (Ig) E, C-C motif chemokine ligand 5 (CCL5, also known as RANTES), CCL11 (also called Eotaxin-1), and IL-17A. In addition, FMT treatments eminently blunted goblet cell hyperplasia and collagen deposition, and remarkably reduced oxidative stress as displayed by decreased reactive oxygen species (ROS), and increased superoxide diamutase (SOD) activity. Furthermore, to clarify the potential mechanisms responsible for the effects, we determined the inflammation and oxidation-related signaling pathway including nuclear factor kappa β (NF-κB), c-Jun N-terminal kinase (JNK), and the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2). FMT treatments appeared to dramatically inhibit the activation of NF-κB and JNK, significantly elevated the expression of heme oxygenase 1 (HO-1) but failed to activate expression of Nrf2. In conclusion, our study suggested that FMT had the therapeutic effects in attenuating airway inflammation and oxidative stress in asthma. VSports手机版.
Keywords: airway inflammation; asthma; c-Jun N-terminal kinase; formononetin; nuclear factor erythroid 2-related factor 2; nuclear factor kappa β; oxidative stress. V体育安卓版.
Copyright © 2020 Yi, Cui, Wang, Tang, Teng, Zhu, Qin, Wuniqiemu, Sun, Wei and Dong. V体育ios版.
Figures



References (VSports手机版)
-
- Aalbers R., Vogelmeier C., Kuna P. (2016). Achieving asthma control with ICS/LABA: A review of strategies for asthma management and prevention. Respir. Med. 111, 1–7. 10.1016/j.rmed.2015.11.002 - DOI (VSports最新版本) - PubMed
-
- Adcock I. M., Caramori G., Chung K. F. (2008). New targets for drug development in asthma. Lancet 372, 1073–1087. 10.1016/S0140-6736(08)61449-X - DOI (V体育ios版) - PubMed
-
- Aladaileh S. H., Hussein O. E., Abukhalil M. H., Saghir S. A. M., Bin-Jumah M., Alfwuaires M. A. (2019). Formononetin Upregulates Nrf2/HO-1 Signaling and Prevents Oxidative Stress, Inflammation, and Kidney Injury in Methotrexate-Induced Rats. Antioxidants 8, 430. 10.3390/antiox8100430 - DOI - PMC - PubMed
-
- Berkman N., Krishnan V. L., Gilbey T., Newton R., O’Connor B., Barnes P. J. (1996). Expression of RANTES mRNA and protein in airways of patients with mild asthma. Am. J. Respir. Crit. Care Med. 154, 1804–1811. 10.1164/ajrccm.154.6.8970374 - V体育官网入口 - DOI - PubMed
LinkOut - more resources (V体育ios版)
Full Text Sources
V体育官网入口 - Research Materials
Miscellaneous

