Neoadjuvant chemotherapy and Avelumab in early stage resectable nonsmall cell lung cancer
- PMID: 32991781
- PMCID: PMC7666740
- DOI: 10.1002/cam4.3456
V体育2025版 - Neoadjuvant chemotherapy and Avelumab in early stage resectable nonsmall cell lung cancer
Abstract
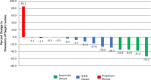
Multiple randomized studies have shown that combination of chemotherapy and immune checkpoint inhibitors (ICIs) leads to better response rates and survival as compared to chemotherapy alone in the advanced stage of NSCLC. Data suggesting a benefit to using ICIs in the neoadjuvant therapy of patients with early stage NSCLC are emerging. Eligible subjects were treatment naïve patients with stage IB, II, and resectable IIIA NSCLC. Patients received three cycles of neoadjuvant chemotherapy with four doses of avelumab every 2 weeks. Patients with squamous cell cancer received cisplatin or carboplatin on day 1 and gemcitabine on days 1 and 8 of each cycle of chemotherapy. Patients with nonsquamous histology received cisplatin or carboplatin with pemetrexed on day 1 of each cycle. Patients then proceeded to their planned surgery. Out of 15 patients accrued as part of stage 1 of the study, four had a radiologic response (1 complete response), lower than the minimum of six responses needed to continue to phase 2 of the study. The study was therefore terminated. Majority had adenocarcinoma histology and stage IIIA disease. The treatment was well tolerated with no unexpected side effects. Four patients (26. 7%) had grade III/IV CTCAE toxicity. This study confirms that the preoperative administration of chemotherapy and avelumab is safe VSports手机版. There was no indication of increased surgical complications. The benefit of adding immunotherapy to chemotherapy did not appear to enhance the overall response rate of patients in the neoadjuvant setting in patients with resectable NSCLC because this study failed to meet its primary endpoint. .
Keywords: immune checkpoint inhibitors; neoadjuvant therapy; nonsmall cell lung cancer; oncogenic drivers V体育安卓版. .
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd. V体育ios版.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non–small‐cell lung cancer. N Engl J Med. 2017;377(20):1919‐1929. - V体育ios版 - PubMed
-
- Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small‐cell lung cancer. N Engl J Med. 2015;373(17):1627‐1639. - PMC (V体育官网入口) - PubMed
-
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous‐cell non–small‐cell lung cancer. N Engl J Med. 2015;373(2):123‐135. - V体育2025版 - PMC - PubMed
-
- Hellmann MD, Paz‐Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small‐cell lung cancer. N Engl J Med. 2019;381(21):2020‐2031. - PubMed
-
- Herbst RS, Baas P, Kim D‐W, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet (London, England). 2016;387(10027):1540‐1550. - PubMed
Publication types
- "V体育ios版" Actions
MeSH terms
- VSports在线直播 - Actions
- "VSports app下载" Actions
- "V体育安卓版" Actions
- Actions (V体育平台登录)
- Actions (VSports注册入口)
- V体育安卓版 - Actions
- Actions (VSports注册入口)
- "VSports手机版" Actions
- "VSports在线直播" Actions
- Actions (VSports手机版)
Substances
- V体育ios版 - Actions
"VSports注册入口" LinkOut - more resources
Full Text Sources
Medical