Miltirone induces cell death in hepatocellular carcinoma cell through GSDME-dependent pyroptosis
- PMID: 32963939
- PMCID: PMC7488361
- DOI: 10.1016/j.apsb.2020.06.015
Miltirone induces cell death in hepatocellular carcinoma cell through GSDME-dependent pyroptosis
Abstract
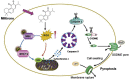
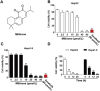
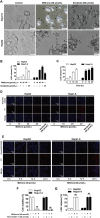
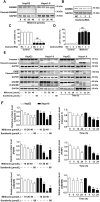
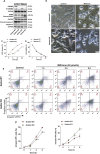
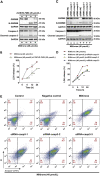
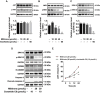
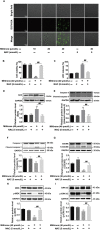
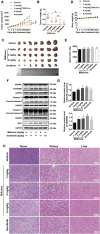
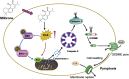
Pyroptosis is a form of programmed cell death, and recently described as a new molecular mechanism of chemotherapy drugs in the treatment of tumors. Miltirone, a derivative of phenanthrene-quinone isolated from the root of Salvia miltiorrhiza Bunge, has been shown to possess anti-cancer activities. Here, we found that miltirone inhibited the cell viability of either HepG2 or Hepa1-6 cells, and induced the proteolytic cleavage of gasdermin E (GSDME) in each hepatocellular carcinoma (HCC) cell line, with concomitant cleavage of caspase 3. Knocking out GSDME switched miltirone-induced cell death from pyroptosis to apoptosis. Additionally, the induction effects of miltirone on GSDME-dependent pyroptosis were attenuated by siRNA-mediated caspase three silencing and the specific caspase three inhibitor Z-DEVD-FMK, respectively. Miltirone effectively elicited intracellular accumulation of reactive oxygen species (ROS), and suppressed phosphorylation of mitogen-activated and extracellular signal-regulated kinase (MEK) and extracellular regulated protein kinases 1/2 (ERK1/2) for pyroptosis induction. Moreover, miltirone significantly inhibited tumor growth and induced pyroptosis in the Hepa1-6 mouse HCC syngeneic model. These results provide a new insight that miltirone is a potential therapeutic agent for the treatment of HCC via GSDME-dependent pyroptosis VSports手机版. .
Keywords: 7-AAD, 7-aminoactinomycin D; AKT, AKT serine/threonine kinase, also known as protein kinase B; ANOVA, analysis of variance; BAX, BCL2-associated X; CCK-8, cell counting kit-8; CRISPR, clustered regularly interspaced short palindromic repeats; Cas9, caspase 9; Cell death; DCFH-DA, dye 2,7-dichlorofluoresce diacetate; DMEM, Dulbecco's modified Eagle's medium; DMSO, dimethyl sulfoxide; ECL, enhanced chemiluminescence; ERK1/2, extracellular regulated protein kinases 1/2; FBS, fetal bovine serum; FITC, fluorescein isothiocyanate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GSDMD, gasdermin D; GSDME; GSDME, gasdermin E; H&E, hematoxylin and eosin; HCC, hepatocellular carcinoma; HRP, horseradish peroxidase; HepG2; Hepa1-6; Hepatocellular carcinoma; IC50, the half maximal inhibitory concentration; IgG (H + L), immunoglobulin G (heavy chain + light chain); KO, knockout; LDH, lactic dehydrogenase; MEK, mitogen-activated and extracellular signal-regulated kinase; MEM, minimum essential medium; MMP, mitochondrial membrane potential; MS, mass spectrum; Miltirone; N-GSDME, N-terminal GSDME; NAC, N-acetyl cysteine; NC, negative control; NMR, nuclear magnetic resonance; NS, no significance; PARP, poly ADP-ribose polymerase; PBS, phosphate-based buffer; PI, propidium iodide; PI3K, phosphatidylinositol 3-kinase; Pyroptosis; RIPA, radioimmunoprecipitation assay; ROS, reactive oxygen species; SD, standard deviation; SDS-PAGE, sodium dodecyl sulphate-polyacrylamide gel electrophoresis; TBST, Tris-buffered saline with Tween solution; TCGA, the Cancer Genome Atlas; VEGF, vascular endothelial growth factor; gRNA, guide RNA; i. p V体育安卓版. , intraperitoneal; i. v. , intravenous; mTOR, mammalian target of rapamycin; p-AKT, phosphorylated-AKT; p-ERK1/2, phosphorylated-ERK1/2; p-MEK, phosphorylated-MEK. .
© 2020 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B V体育ios版. V. .
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
VSports最新版本 - References
-
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Keating G.M., Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223–240. - PubMed
-
- Augello G., Emma M.R., Cusimano A., Azzolina A., Mongiovì S., Puleio R. Targeting HSP90 with the small molecule inhibitor AUY922 (luminespib) as a treatment strategy against hepatocellular carcinoma. Int J Canc. 2019;144:2613–2624. - PubMed
-
- Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
-
- Cheng A.L., Kang Y.K., Chen Z.D., Tsao C.J., Qin S.K., Kim J.S. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomized, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. - PubMed (V体育官网入口)
"V体育官网入口" LinkOut - more resources
Full Text Sources
"V体育2025版" Research Materials
"VSports最新版本" Miscellaneous

