A Multifunctional Role of Leucine-Rich α-2-Glycoprotein 1 in Cutaneous Wound Healing Under Normal and Diabetic Conditions
- PMID: 32887674
- PMCID: PMC7576570
- DOI: 10.2337/db20-0585 (V体育ios版)
A Multifunctional Role of Leucine-Rich α-2-Glycoprotein 1 in Cutaneous Wound Healing Under Normal and Diabetic Conditions
"V体育官网" Abstract
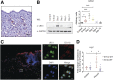
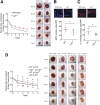
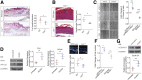
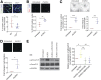
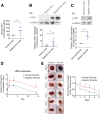
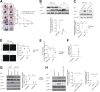
Delayed wound healing is commonly associated with diabetes. It may lead to amputation and death if not treated in a timely fashion. Limited treatments are available partially due to the poor understanding of the complex disease pathophysiology. Here, we investigated the role of leucine-rich α-2-glycoprotein 1 (LRG1) in normal and diabetic wound healing. First, our data showed that LRG1 was significantly increased at the inflammation stage of murine wound healing, and bone marrow-derived cells served as a major source of LRG1. LRG1 deletion causes impaired immune cell infiltration, reepithelialization, and angiogenesis. As a consequence, there is a significant delay in wound closure VSports手机版. On the other hand, LRG1 was markedly induced in diabetic wounds in both humans and mice. LRG1-deficient mice were resistant to diabetes-induced delay in wound repair. We further demonstrated that this could be explained by the mitigation of increased neutrophil extracellular traps (NETs) in diabetic wounds. Mechanistically, LRG1 mediates NETosis in an Akt-dependent manner through TGFβ type I receptor kinase ALK5. Taken together, our studies demonstrated that LRG1 derived from bone marrow cells is required for normal wound healing, revealing a physiological role for this glycoprotein, but that excess LRG1 expression in diabetes is pathogenic and contributes to chronic wound formation. .
© 2020 by the American Diabetes Association V体育安卓版. .
Figures

References
-
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321 - PubMed
-
- Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376:2367–2375 - VSports在线直播 - PubMed
-
- Gregg EW, Li Y, Wang J, et al. . Changes in diabetes-related complications in the United States, 1990-2010. N Engl J Med 2014;370:1514–1523 - PubMed
-
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 - PubMed (V体育平台登录)
Publication types
- V体育2025版 - Actions
MeSH terms
- Actions (VSports注册入口)
- Actions (VSports手机版)
- "V体育安卓版" Actions
- Actions (VSports app下载)
- "V体育安卓版" Actions
- "V体育官网入口" Actions
- Actions (VSports在线直播)
- V体育平台登录 - Actions
- "VSports app下载" Actions
- VSports最新版本 - Actions
- Actions (VSports app下载)
- "VSports app下载" Actions
- Actions (V体育官网入口)
- V体育官网入口 - Actions
Substances
- "VSports在线直播" Actions
- "VSports注册入口" Actions
- Actions (VSports)
Associated data (VSports在线直播)
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
V体育平台登录 - Research Materials
Miscellaneous

