The Role of Necroptosis in ROS-Mediated Cancer Therapies and Its Promising Applications
- PMID: 32764483
- PMCID: PMC7465132
- DOI: 10.3390/cancers12082185
"VSports app下载" The Role of Necroptosis in ROS-Mediated Cancer Therapies and Its Promising Applications
Abstract
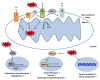
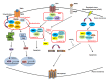
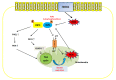
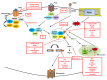
Over the past decades, promising therapies targeting different signaling pathways have emerged. Among these pathways, apoptosis has been well investigated and targeted to design diverse chemotherapies. However, some patients are chemoresistant to these therapies due to compromised apoptotic cell death. Hence, exploring alternative treatments aimed at different mechanisms of cell death seems to be a potential strategy for bypassing impaired apoptotic cell death VSports手机版. Emerging evidence has shown that necroptosis, a caspase-independent form of cell death with features between apoptosis and necrosis, can overcome the predicament of drug resistance. Furthermore, previous studies have also indicated that there is a close correlation between necroptosis and reactive oxygen species (ROS); both necroptosis and ROS play significant roles both under human physiological conditions such as the regulation of inflammation and in cancer biology. Several small molecules used in experiments and clinical practice eliminate cancer cells via the modulation of ROS and necroptosis. The molecular mechanisms of these promising therapies are discussed in detail in this review. .
Keywords: cancer; chemotherapy; necroptosis; reactive oxygen species. V体育安卓版.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
References
-
- Su Z., Yang Z., Xie L., DeWitt J.P., Chen Y. Cancer therapy in the necroptosis era. Cell Death Differ. 2016;23:748–756. doi: 10.1038/cdd.2016.8. - DOI (VSports) - PMC - PubMed
-
- Sethi G., Shanmugam M.K., Warrier S., Merarchi M., Arfuso F., Kumar A.P., Bishayee A. Pro-Apoptotic and Anti-Cancer Properties of Diosgenin: A Comprehensive and Critical Review. Nutrients. 2018;10:645. doi: 10.3390/nu10050645. - "V体育平台登录" DOI - PMC - PubMed
-
- Li Y., Wang X., Cheng S., Du J., Deng Z., Zhang Y., Liu Q., Gao J., Cheng B., Ling C. Diosgenin induces G2/M cell cycle arrest and apoptosis in human hepatocellular carcinoma cells. Oncol. Rep. 2015;33:693–698. doi: 10.3892/or.2014.3629. - DOI (V体育官网) - PubMed
-
- Srinivasan S., Koduru S., Kumar R., Venguswamy G., Kyprianou N., Damodaran C. Diosgenin targets Akt-mediated prosurvival signaling in human breast cancer cells. Int. J. Cancer. 2009;125:961–967. doi: 10.1002/ijc.24419. - "V体育ios版" DOI - PubMed
Publication types
- Actions (VSports在线直播)
Grants and funding (V体育官网入口)
LinkOut - more resources
Full Text Sources
Other Literature Sources (V体育平台登录)
Miscellaneous

