Bile acids regulate intestinal antigen presentation and reduce graft-versus-host disease without impairing the graft-versus-leukemia effect
- PMID: 32675222
- PMCID: PMC8327708 (V体育ios版)
- DOI: 10.3324/haematol.2019.242990
Bile acids regulate intestinal antigen presentation and reduce graft-versus-host disease without impairing the graft-versus-leukemia effect
Abstract
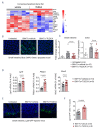
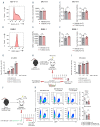
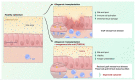
Acute graft-versus-host disease causes significant mortality in patients undergoing allogeneic hematopoietic cell transplantation. Immunosuppressive treatment for graft-versus-host disease can impair the beneficial graft-versus-leukemia effect and facilitate malignancy relapse. Therefore, novel approaches that protect and regenerate injured tissues without impeding the donor immune system are needed. Bile acids regulate multiple cellular processes and are in close contact with the intestinal epithelium, a major target of acute graft-versus-host disease. Here, we found that the bile acid pool is reduced following graft-versus-host disease induction in a preclinical model. We evaluated the efficacy of bile acids to protect the intestinal epithelium without reducing anti-tumor immunity. We observed that application of bile acids decreased cytokine-induced cell death in intestinal organoids and cell lines. Systemic prophylactic administration of tauroursodeoxycholic acid, the most potent compound in our in vitro studies, reduced graft-versus-host disease severity in three different murine transplantation models. This effect was mediated by decreased activity of the antigen presentation machinery and subsequent prevention of apoptosis of the intestinal epithelium. Moreover, bile acid administration did not alter the bacterial composition in the intestine suggesting that its effects are cell-specific and independent of the microbiome. Treatment of human and murine leukemic cell lines with tauroursodeoxycholic acid did not interfere with the expression of antigen presentation-related molecules. Systemic T cell expansion and especially their cytotoxic capacity against leukemic cells remained intact. This study establishes a role for bile acids in the prevention of acute graft-versus-host disease without impairing the graft-versus-leukemia effect. In particular, we provide a scientific rationale for the systematic use of tauroursodeoxycholic acid in patients undergoing allogeneic hematopoietic cell transplantation VSports手机版. .
"VSports注册入口" Figures





References
-
- de Lima M, Porter DL, Battiwalla M, et al. . Proceedings from the National Cancer Institute's Second International Workshop on the biology, prevention, and treatment of relapse after hematopoietic stem cell transplantation: part III. Prevention and treatment of relapse after allogeneic transplantation. Biol Blood Marrow Transplant. 2014;20(1):4-13. - PMC - PubMed
-
- Zeiser R, Blazar BR. Acute Graft-versus- Host Disease - Biologic Process, Prevention, and Therapy. N Engl J Med. 2017; 377(22):2167-2179. - VSports在线直播 - PMC - PubMed
-
- McDonald GB. How I treat acute graft-versus- host disease of the gastrointestinal tract and the liver. Blood. 2016;127(12):1544-1550. - "V体育2025版" PMC - PubMed
-
- Takashima S, Kadowaki M, Aoyama K, et al. . The Wnt agonist R-spondin1 regulates systemic graft-versus-host disease by protecting intestinal stem cells. J Exp Med. 2011;208(2):285-294. - V体育安卓版 - PMC - PubMed
-
- Eriguchi Y, Takashima S, Oka H, et al. . Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of alpha-defensins. Blood. 2012;120(1):223-231. - PubMed
Publication types
- "VSports app下载" Actions
MeSH terms
- VSports app下载 - Actions
- "VSports app下载" Actions
- VSports app下载 - Actions
- Actions (VSports)
Substances
- "V体育官网入口" Actions
LinkOut - more resources
Full Text Sources
VSports在线直播 - Medical
Molecular Biology Databases

