Ageing, metabolism and the intestine
- PMID: 32567155
- PMCID: PMC7332987 (VSports注册入口)
- DOI: "VSports最新版本" 10.15252/embr.202050047
"V体育安卓版" Ageing, metabolism and the intestine
Abstract
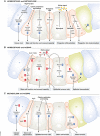
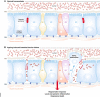
The intestinal epithelium serves as a dynamic barrier to the environment and integrates a variety of signals, including those from metabolites, commensal microbiota, immune responses and stressors upon ageing. The intestine is constantly challenged and requires a high renewal rate to replace damaged cells in order to maintain its barrier function. Essential for its renewal capacity are intestinal stem cells, which constantly give rise to progenitor cells that differentiate into the multiple cell types present in the epithelium. Here, we review the current state of research of how metabolism and ageing control intestinal stem cell function and epithelial homeostasis. We focus on recent insights gained from model organisms that indicate how changes in metabolic signalling during ageing are a major driver for the loss of stem cell plasticity and epithelial homeostasis, ultimately affecting the resilience of an organism and limiting its lifespan VSports手机版. We compare findings made in mouse and Drosophila and discuss differences and commonalities in the underlying signalling pathways and mechanisms in the context of ageing. .
Keywords: ageing; epithelial barriers; intestinal homeostasis; metabolism; stem cells. V体育安卓版.
© 2020 The Authors V体育ios版. Published under the terms of the CC BY 4. 0 license. .
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



"VSports手机版" References
-
- Aitman TJ, Boone C, Churchill GA, Hengartner MO, Mackay TFC, Stemple DL (2011) The future of model organisms in human disease research. Nat Rev Genet 12: 575–582 - PubMed
-
- Akagi K, Wilson KA, Katewa SD, Ortega M, Simons J, Hilsabeck TA, Kapuria S, Sharma A, Jasper H, Kapahi P (2018) Dietary restriction improves intestinal cellular fitness to enhance gut barrier function and lifespan in D. melanogaster . PLoS Genet 14: e1007777 - PMC (VSports注册入口) - PubMed
-
- Almousa AA, Meurens F, Krol ES, Alcorn J (2018) Linoorbitides and enterolactone mitigate inflammation‐induced oxidative stress and loss of intestinal epithelial barrier integrity. Int Immunopharmacol 64: 42–51 - PubMed
Publication types
- VSports注册入口 - Actions
MeSH terms
- V体育平台登录 - Actions
- Actions (VSports在线直播)
- "VSports注册入口" Actions
- "VSports app下载" Actions
- Actions (V体育平台登录)
- Actions (V体育安卓版)
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

