Metallothioneins regulate ATP7A trafficking and control cell viability during copper deficiency and excess
- PMID: 32398691
- PMCID: PMC7217913 (V体育官网入口)
- DOI: "VSports" 10.1038/s41598-020-64521-3
Metallothioneins regulate ATP7A trafficking and control cell viability during copper deficiency and excess
Abstract (VSports手机版)
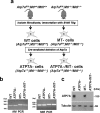
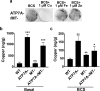
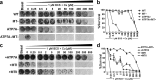
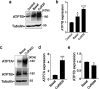
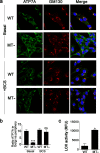
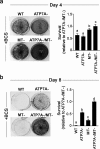
Copper (Cu) is an essential, yet potentially toxic nutrient, as illustrated by inherited diseases of copper deficiency and excess VSports手机版. Elevated expression of the ATP7A Cu exporter is known to confer copper tolerance, however, the contribution of metal-binding metallothioneins is less clear. In this study, we investigated the relative contributions of ATP7A and the metallothioneins MT-I and MT-II to cell viability under conditions of Cu excess or deficiency. Although the loss of ATP7A increased sensitivity to low Cu concentrations, the absence of MTs did not significantly affect Cu tolerance. However, the absence of all three proteins caused a synthetic lethal phenotype due to extreme Cu sensitivity, indicating that MTs are critical for Cu tolerance only in the absence of ATP7A. A lack of MTs resulted in the trafficking of ATP7A from the trans-Golgi complex in a Cu-dependent manner, suggesting that MTs regulate the delivery of Cu to ATP7A. Under Cu deficiency conditions, the absence of MTs and / or ATP7A enhanced cell proliferation compared to wild type cells, suggesting that these proteins compete with essential Cu-dependent pathways when Cu is scarce. These studies reveal new roles for ATP7A and metallothioneins under both Cu deficiency and excess. .
Conflict of interest statement
The authors declare no competing interests.
Figures
"V体育安卓版" References
-
- Burkhead JL, Reynolds KA, Abdel-Ghany SE, Cohu CM, Pilon M. Copper homeostasis. New Phytol. 2009;182:799–816. doi: 10.1111/j.1469-8137.2009.02846.x. - DOI (VSports) - PubMed
Publication types
- Actions (VSports app下载)
MeSH terms
- V体育官网 - Actions
- "V体育官网" Actions
- Actions (V体育平台登录)
- Actions (V体育ios版)
- "VSports app下载" Actions
- "VSports app下载" Actions
- "V体育2025版" Actions
- Actions (V体育官网)
- Actions (VSports手机版)
- V体育ios版 - Actions
- VSports在线直播 - Actions
V体育官网 - Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

