Electrical stimulation inhibits Val-boroPro-induced pyroptosis in THP-1 macrophages via sirtuin3 activation to promote autophagy and inhibit ROS generation
- PMID: 32289749
- PMCID: PMC7185124
- DOI: 10.18632/aging.103038
Electrical stimulation inhibits Val-boroPro-induced pyroptosis in THP-1 macrophages via sirtuin3 activation to promote autophagy and inhibit ROS generation
Abstract (V体育平台登录)
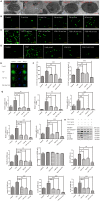
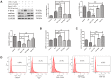
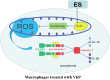
The incidence of atherosclerosis (AS), a major contributor to cardiovascular disease, is steadily rising along with an increasingly older population worldwide. Pyroptosis, a form of inflammatory programmed cell death, determines the release of pro-inflammatory mediators by endothelial cells, smooth muscle cells, and atheroma-associated macrophages and foam cells, thereby playing a critical role in AS progression. Canonical pyroptosis is mediated by inflammasome formation, activation of caspase-1, and maturation and release of proinflammatory cytokines. Electrical stimulation (ES) is a noninvasive, safe therapy that has been shown to alleviate symptoms in several health conditions VSports手机版. Here, we investigated the anti-inflammatory and anti-pyroptotic effects of ES in human THP-1 macrophages treated with the dipeptidyl peptidase inhibitor Val-boroPro (VbP). We found that ES downregulated NOD-like receptor family protein 3 (NLRP3) inflammasome, ASC, and caspase-1 expression and abrogated the release of Interleukin-1β (IL-1β) and Interleukin-18 (IL-18), indicating effective pyroptosis inhibition. These changes were paralleled by a reduction in reactive oxygen species (ROS) production, reversal of VbP-induced sirtuin3 (Sirt3) downregulation, deacetylation of ATG5, and induction of autophagy. These findings suggest that ES may be a viable strategy to counteract pyroptosis-mediated inflammation in AS by raising Sirt3 to promote autophagy and inhibit ROS generation. .
Keywords: ROS; electrical stimulation; macrophages; pyroptosis; sirtuin3 V体育安卓版. .
Conflict of interest statement
Figures






"V体育官网" References
-
- Wang JC, Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ Res. 2012; 111:245–59. 10.1161/CIRCRESAHA.111.261388 - DOI (V体育2025版) - PubMed
-
- Sato K, Yoshizawa H, Seki T, Shirai R, Yamashita T, Okano T, Shibata K, Wakamatsu MJ, Mori Y, Morita T, Matsuyama TA, Ishibashi-Ueda H, Hirano T, Watanabe T. Chemerin-9, a potent agonist of chemerin receptor (ChemR23), prevents atherogenesis. Clin Sci (Lond). 2019; 133:1779–96. 10.1042/CS20190336 - VSports最新版本 - DOI - PubMed
Publication types
"V体育官网" MeSH terms
- V体育安卓版 - Actions
- Actions (V体育ios版)
- V体育2025版 - Actions
- "V体育官网入口" Actions
- V体育安卓版 - Actions
- "VSports注册入口" Actions
- V体育官网 - Actions
Substances
- "V体育平台登录" Actions
- V体育官网入口 - Actions
- "VSports" Actions
- VSports - Actions
- "VSports最新版本" Actions
- "V体育官网" Actions
LinkOut - more resources
Full Text Sources
Medical
Research Materials
VSports - Miscellaneous

