Antibody Response against Cowpea Mosaic Viral Nanoparticles Improves In Situ Vaccine Efficacy in Ovarian Cancer
- PMID: 32133838
- PMCID: PMC8085886
- DOI: 10.1021/acsnano.9b07865
Antibody Response against Cowpea Mosaic Viral Nanoparticles Improves In Situ Vaccine Efficacy in Ovarian Cancer (V体育2025版)
VSports在线直播 - Abstract
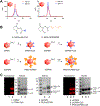
Cancer immunotherapies are designed to facilitate recognition and elimination of transformed cells by the immune system. We have established the immunotherapeutic efficacy of the plant virus cowpea mosaic virus (CPMV) as an in situ vaccine in several syngeneic tumor mouse models as well as in companion dogs with metastatic melanoma. Intratumoral injection of CPMV modulates the local tumor microenvironment to relieve immunosuppression and potentiate antitumor immunity VSports手机版. The viral nucleocapsid that drives this antitumor immunity, however, also is a potent immunogen itself, and thus immune response in the form of anti-CPMV antibodies is expected during the treatment based on repeat administrations. Moreover, being part of the food chain, pre-existing antibodies to plant viruses may be prevalent. The presence of such pre-existing anti-CPMV immunity could potentially impact immunotherapeutic efficacy of the in situ vaccine and could have translational implications. To address such concerns, this study evaluated the efficacy of CPMV in situ vaccine in the presence of pre-existing antibodies in a syngeneic mouse model of ovarian cancer. Our results indicate that prior exposure to CPMV had no negative impact on the efficacy of CPMV in situ vaccine. Strikingly, an improved efficacy of CPMV in situ vaccine was observed. This study therefore presents an important milestone in the translational development of plant viral-based in situ vaccines and alleviates concerns about the presence of anti-CPMV antibodies, which are developed during the course of treatment but have no impact on immunotherapeutic efficacy. .
Keywords: CPMV; antiviral antibodies; cancer immunotherapy; in situ vaccine; ovarian cancer; plant viruses V体育安卓版. .
Conflict of interest statement (V体育安卓版)
The authors declare no competing financial interest.
Figures

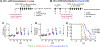


References
-
- Joyce JA; Fearon DT T Cell Exclusion, Immune Privilege, and the Tumor Microenvironment. Science 2015, 348, 74–80. - "VSports注册入口" PubMed
-
- Le QV; Suh J; Choi JJ; Park GT; Lee JW; Shim G; Oh YK In Situ Nanoadjuvant-Assembled Tumor Vaccine for Preventing Long-Term Recurrence. ACS Nano 2019, 13, 7442–7462. - PubMed
-
- Bommareddy PK; Kaufman HL Unleashing the Therapeutic Potential of Oncolytic Viruses. J. Clin. Invest 2018, 128, 1258–1260. - VSports最新版本 - PMC - PubMed
"V体育官网入口" Publication types
MeSH terms
- "V体育官网入口" Actions
- Actions (V体育官网入口)
- "VSports注册入口" Actions
- "VSports注册入口" Actions
- "V体育2025版" Actions
"VSports app下载" Substances
- V体育ios版 - Actions
- Actions (V体育安卓版)
Grants and funding
LinkOut - more resources (V体育官网入口)
Full Text Sources
Medical

