The Use of Both Therapeutic and Prophylactic Vaccines in the Therapy of Papillomavirus Disease
- PMID: 32133000
- PMCID: PMC7040023
- DOI: 10.3389/fimmu.2020.00188
The Use of Both Therapeutic and Prophylactic Vaccines in the Therapy of Papillomavirus Disease
"VSports最新版本" Abstract
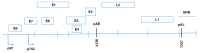
Human papillomavirus (HPV) is the most common sexually transmitted virus. The high-risk HPV types (i. e. , HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59) are considered to be the main etiological agents of genital tract cancers, such as cervical, vulvar, vaginal, penile, and anal cancers, and of a subset of head and neck cancers. Three prophylactic HPV vaccines are available that are bivalent (vs. HPV16, 18), tetravalent (vs. HPV6, 11, 16, 18), and non-avalent (vs. HPV6, 11, 16, 18, 31, 33,45, 52, 58) VSports手机版. All of these vaccines are based on recombinant DNA technology, and they are prepared from the purified L1 protein that self-assembles to form the HPV type-specific empty shells (i. e. , virus-like particles). These vaccines are highly immunogenic and induce specific antibodies. Therapeutic vaccines differ from prophylactic vaccines, as they are designed to generate cell-mediated immunity against transformed cells, rather than neutralizing antibodies. Among the HPV proteins, the E6 and E7 oncoproteins are considered almost ideal as targets for immunotherapy of cervical cancer, as they are essential for the onset and evolution of malignancy and are constitutively expressed in both premalignant and invasive lesions. Several strategies have been investigated for HPV therapeutic vaccines designed to enhance CD4+ and CD8+ T-cell responses, including genetic vaccines (i. e. , DNA/ RNA/virus/ bacterial), and protein-based, peptide-based or dendritic-cell-based vaccines. However, no vaccine has yet been licensed for therapeutic use. Several studies have suggested that administration of prophylactic vaccines immediately after surgical treatment of CIN2 cervical lesions can be considered as an adjuvant to prevent reactivation or reinfection, and other studies have described the relevance of prophylactic vaccines in the management of genital warts. This review summarizes the leading features of therapeutic vaccines, which mainly target the early oncoproteins E6 and E7, and prophylactic vaccines, which are based on the L1 capsid protein. Through an analysis of the specific immunogenic properties of these two types of vaccines, we discuss why and how prophylactic vaccines can be effective in the treatment of HPV-related lesions and relapse. .
Keywords: cancer; human papillomavirus; immune response; prophylactic vaccine; therapeutic vaccine V体育安卓版. .
Copyright © 2020 Garbuglia, Lapa, Sias, Capobianchi and Del Porto V体育ios版. .
Figures
References
-
- Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet. (2013) 382:889–99. 10.1016/S0140-6736(13)60022-7 - V体育官网入口 - DOI - PubMed
-
- Vaccarella S, Franceschi S, Engholm G, Lönnberg S, Khan S, Bray F. 50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidence. Br J Cancer. (2014) 111:965–9. 10.1038/bjc.2014.362 - DOI (VSports注册入口) - PMC - PubMed
Publication types
- Actions (VSports在线直播)
MeSH terms
- VSports在线直播 - Actions
- VSports手机版 - Actions
- Actions (VSports app下载)
- V体育官网入口 - Actions
- Actions (V体育2025版)
- Actions (V体育官网入口)
- "VSports注册入口" Actions
- "VSports app下载" Actions
- V体育平台登录 - Actions
- "V体育官网" Actions
- Actions (V体育安卓版)
Substances
- Actions (V体育安卓版)
"VSports" LinkOut - more resources
Full Text Sources
VSports - Medical
Research Materials