Temporal changes in gut microbiota profile in children with acute lymphoblastic leukemia prior to commencement-, during-, and post-cessation of chemotherapy (VSports注册入口)
- PMID: 32093640
- PMCID: PMC7041273 (VSports手机版)
- DOI: 10.1186/s12885-020-6654-5
Temporal changes in gut microbiota profile in children with acute lymphoblastic leukemia prior to commencement-, during-, and post-cessation of chemotherapy
VSports app下载 - Abstract
Background: Alteration in gut microbiota has been recently linked with childhood leukemia and the use of chemotherapy. Whether the perturbed microbiota community is restored after disease remission and cessation of cancer treatment has not been evaluated. This study examines the chronological changes of gut microbiota in children with acute lymphoblastic leukemia (ALL) prior to the start-, during-, and following cessation of chemotherapy VSports手机版. .
Methodology: We conducted a longitudinal observational study in gut microbiota profile in a group of paediatric patients diagnosed with ALL using 16 s ribosomal RNA sequencing and compared these patients' microbiota pattern with age and ethnicity-matched healthy children V体育安卓版. Temporal changes of gut microbiota in these patients with ALL were also examined at different time-points in relation to chemotherapy. .
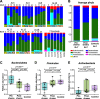
Results: Prior to commencement of chemotherapy, gut microbiota in children with ALL had larger inter-individual variability compared to healthy controls and was enriched with bacteria belonging to Bacteroidetes phylum and Bacteroides genus. The relative abundance of Bacteroides decreased upon commencement of chemotherapy. Restitution of gut microbiota composition to resemble that of healthy controls occurred after cessation of chemotherapy. However, the microbiota composition (beta diversity) remained distinctive and a few bacteria were different in abundance among the patients with ALL compared to controls despite completion of chemotherapy and presumed restoration of normal health. V体育ios版.
Conclusion: Our findings in this pilot study is the first to suggest that gut microbiota profile in children with ALL remains marginally different from healthy controls even after cessation of chemotherapy. These persistent microbiota changes may have a role in the long-term wellbeing in childhood cancer survivors but the impact of these changes in subsequent health perturbations in these survivors remain unexplored. VSports最新版本.
Keywords: Bacteroides; Bacteroidetes; Chemotherapy; Childhood acute lymphoblastic leukemia; Microbiome; Microbiota dysbiosis. V体育平台登录.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol. 2015;31(1):69–75. doi: 10.1097/MOG.0000000000000139. - "VSports手机版" DOI - PMC - PubMed
-
- Sanchez B, Delgado S, Blanco-Miguez A, Lourenco A, Gueimonde M, Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res. 2017;61(1):1600240. doi: 10.1002/mnfr.201600240. - DOI (V体育平台登录) - PubMed
MeSH terms
- "VSports注册入口" Actions
- "VSports手机版" Actions
- "V体育ios版" Actions
- VSports在线直播 - Actions
- Actions (VSports app下载)
- Actions (V体育平台登录)
- V体育官网 - Actions
- "VSports" Actions
- "VSports在线直播" Actions
Substances
Grants and funding
LinkOut - more resources (V体育平台登录)
Full Text Sources
Molecular Biology Databases

