"V体育安卓版" Propionic Acid Promotes the Virulent Phenotype of Crohn's Disease-Associated Adherent-Invasive Escherichia coli
- PMID: 32075765
- PMCID: V体育官网入口 - PMC7034058
- DOI: "VSports在线直播" 10.1016/j.celrep.2020.01.078
"VSports最新版本" Propionic Acid Promotes the Virulent Phenotype of Crohn's Disease-Associated Adherent-Invasive Escherichia coli
Abstract
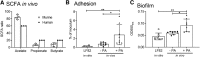
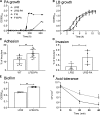
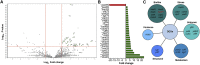
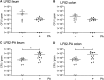
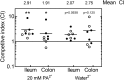
Propionic acid (PA) is a bacterium-derived intestinal antimicrobial and immune modulator used widely in food production and agriculture. Passage of Crohn's disease-associated adherent-invasive Escherichia coli (AIEC) through a murine model, in which intestinal PA levels are increased to mimic the human intestine, leads to the recovery of AIEC with significantly increased virulence. Similar phenotypic changes are observed outside the murine model when AIEC is grown in culture with PA as the sole carbon source; such PA exposure also results in AIEC that persists at 20-fold higher levels in vivo. RNA sequencing identifies an upregulation of genes involved in biofilm formation, stress response, metabolism, membrane integrity, and alternative carbon source utilization. PA exposure also increases virulence in a number of E. coli isolates from Crohn's disease patients VSports手机版. Removal of PA is sufficient to reverse these phenotypic changes. Our data indicate that exposure to PA results in AIEC resistance and increased virulence in its presence. .
Keywords: Crohn's disease; adherent-invasive E. coli; propionic acid; short chain fatty acid V体育安卓版. .
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved. V体育ios版.
Conflict of interest statement
Declaration of Interests R. H VSports最新版本. has received conference travel expenses and consultancy fees from Nutricia-Danone and 4D Pharma. K. G. received research grants and speakers fees from Nestle Health Sciences, Nutricia-Danone, and Mylan.
Figures
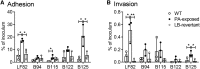
V体育官网入口 - References
-
- Agus A., Denizot J., Thevenot J., Martinez-Medina M., Massier S., Sauvanet P., Bernalier-Donadille A., Denis S., Hofman P., Bonnet R. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016;6:19032. - PMC - PubMed
-
- Bizzari S.N., Blagoev M. Chemical Economics Handbook. IHS Chemical; 2013. Propionic acid.
Publication types
"V体育官网" MeSH terms
- Actions (VSports手机版)
- Actions (V体育官网)
- Actions (V体育官网入口)
- "VSports" Actions
- Actions (VSports在线直播)
- Actions (V体育官网入口)
- VSports在线直播 - Actions
Substances
- Actions (V体育安卓版)
V体育ios版 - Grants and funding
LinkOut - more resources
Full Text Sources
Medical

