V体育平台登录 - LncRNA MEG3 targeting miR-424-5p via MAPK signaling pathway mediates neuronal apoptosis in ischemic stroke
- PMID: 32065781
- PMCID: PMC7066902
- DOI: "V体育官网" 10.18632/aging.102790
LncRNA MEG3 targeting miR-424-5p via MAPK signaling pathway mediates neuronal apoptosis in ischemic stroke
Abstract
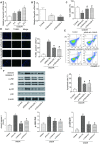
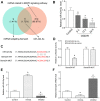
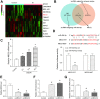
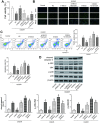
Emerging evidence suggests that long non-coding RNAs (lncRNAs) are significant regulators in the pathological process of ischemic stroke (IS). However, little is known about lncRNAs and their roles in IS. In this study, we aimed to screen out differentially expressed lncRNAs and revealed the underlying mechanisms in IS. The results of bioinformatic analysis showed that lncRNA MEG3 and Sema3A were over-expressed in IS samples, while miR-424-5p was lower-expressed. Correlation between MEG3/miR-424-5p, and miR-424-5p/Sema3A were predicted with miRanda and TargetScan, and verified by dual luciferase assay. Inhibition of MEG3 remarkably increased the expression of miR-424-5p and decreased the expression of Sema3A, which also led to in an increased cell viability and decreased cellular apoptosis in oxygen-glucose deprivation and reoxygenation (OGD/R) model, as well as an activated MAPK signaling pathways. Consistently, MEG3 was upregulated in MCAO mice, knockdown of MEG3 reduced the infarct volume and improved neurobehavioral outcomes in rats following MCAO. In conclusion, it was demonstrated that MEG3 accelerated the process of IS by suppressing miR-424-5p, which targeted Sema3A and the activated MAPK pathway. These results might provide useful information for exploring the potential therapeutic targets in IS. VSports手机版.
Keywords: MEG3; Sema3A; ischemic stroke; miR-424-5p. V体育安卓版.
Conflict of interest statement
Figures
V体育官网入口 - References
-
- Bao MH, Szeto V, Yang BB, Zhu SZ, Sun HS, Feng ZP. Long non-coding RNAs in ischemic stroke. Cell Death Dis. 2018; 9:281. 10.1038/s41419-018-0282-x - V体育官网 - DOI - PMC - PubMed
-
- Randolph SA. Ischemic Stroke. Workplace Health Saf. 2016; 64:444. 10.1177/2165079916665400 - V体育2025版 - DOI - PubMed
-
- Young RS, Ponting CP. Identification and function of long non-coding RNAs. Essays Biochem. 2013; 54:113–26. 10.1042/bse0540113 - DOI (V体育官网) - PubMed
-
- Dykstra-Aiello C, Jickling GC, Ander BP, Shroff N, Zhan X, Liu D, Hull H, Orantia M, Stamova BS, Sharp FR. Altered Expression of Long Noncoding RNAs in Blood After Ischemic Stroke and Proximity to Putative Stroke Risk Loci. Stroke. 2016; 47:2896–903. 10.1161/STROKEAHA.116.013869 - DOI - PMC - PubMed
"VSports手机版" Publication types
- V体育ios版 - Actions
MeSH terms
- "VSports注册入口" Actions
- Actions (V体育官网)
- Actions (VSports注册入口)
- VSports手机版 - Actions
- "V体育官网" Actions
- Actions (VSports在线直播)
- V体育官网入口 - Actions
- Actions (VSports app下载)
- "VSports注册入口" Actions
Substances
- Actions (VSports注册入口)
LinkOut - more resources
Full Text Sources
"V体育2025版" Medical

