Leaky-gut enhanced lupus progression in the Fc gamma receptor-IIb deficient and pristane-induced mouse models of lupus
- PMID: 31964918
- PMCID: PMC6972921
- DOI: 10.1038/s41598-019-57275-0
Leaky-gut enhanced lupus progression in the Fc gamma receptor-IIb deficient and pristane-induced mouse models of lupus
Abstract
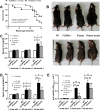
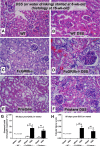
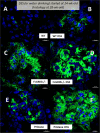
The influence of gut-leakage or gut-microbiota upon lupus progression was explored in 2 lupus mouse models. Pristane, administered in 4-wk-old wild-type (WT) female mice, induced lupus characteristics at 24-wk-old similar to the lupus-onset in FcGRIIb-/- mice. Gut-microbiota alteration was induced by co-housing together with the gavage of feces from 40-wk-old FcGRIIb-/- mice (symptomatic lupus). On the other hand, gut-leakage was induced by dextran sulfate solution (DSS) VSports手机版. DSS and gut-microbiota alteration induced high serum anti-dsDNA immunoglobulin (Ig) as early as 30 days post-DSS only in FcGRIIb-/- mice. DSS, but not gut-microbiota alteration, enhanced lupus characteristics (serum creatinine and proteinuria) in both lupus models (but not in WT) at 60 days post-DSS. Indeed, DSS induced the translocation of molecular components of gut-pathogens as determined by bacterial burdens in mesenteric lymph node (MLN), endotoxemia (gut-bacterial molecule) and serum (1→3)-β-D-glucan (BG) (gut-fungal molecule) as early as 15 days post-DSS together with enhanced MLN apoptosis in both WT and lupus mice. However, DSS induced spleen apoptosis in FcGRIIb-/- and WT mice at 30 and 60 days post-DSS, respectively, suggesting the higher impact of gut-leakage against spleen of lupus mice. In addition, macrophages preconditioning with LPS plus BG were susceptible to starvation-induced apoptosis, predominantly in FcGRIIb-/- cell, implying the influence of gut-leakage upon cell stress. In summary, gut-leakage induced gut-translocation of organismal-molecules then enhanced the susceptibility of stress-induced apoptosis, predominantly in lupus. Subsequently, the higher burdens of apoptosis in lupus mice increased anti-dsDNA Ig and worsen lupus severity through immune complex deposition. Hence, therapeutic strategies addressing gut-leakage in lupus are interesting. .
Conflict of interest statement
The authors declare no competing interests.
Figures











References
-
- Tsokos GC. Systemic Lupus Erythematosus. New England Journal of Medicine. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. - V体育官网 - DOI - PubMed
-
- Tsuchiya N, Kyogoku C. Role of Fcγ receptor IIb polymorphism in the genetic background of systemic lupus erythematosus: Insights from Asia. Autoimmunity. 2005;38:347–352. doi: 10.1080/08916930500123926. - DOI (V体育官网) - PubMed
-
- Clatworthy MR, et al. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malaria. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7169–7174. doi: 10.1073/pnas.0608889104. - "VSports app下载" DOI - PMC - PubMed
Publication types
- "V体育安卓版" Actions
"VSports app下载" MeSH terms
- "V体育官网" Actions
- V体育官网入口 - Actions
- V体育安卓版 - Actions
- "VSports app下载" Actions
Substances
- "VSports在线直播" Actions
- VSports最新版本 - Actions
- Actions (V体育官网)
LinkOut - more resources
Full Text Sources
Medical (V体育ios版)
Molecular Biology Databases (V体育平台登录)

