High-parametric evaluation of human invariant natural killer T cells to delineate heterogeneity in allo- and autoimmunity
- PMID: 31935280
- PMCID: PMC7068034
- DOI: 10.1182/blood.2019001903
High-parametric evaluation of human invariant natural killer T cells to delineate heterogeneity in allo- and autoimmunity
Abstract
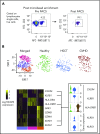
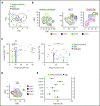
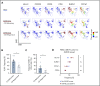
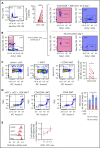
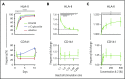
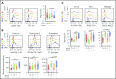
Human invariant natural killer T (iNKT) cells are a rare innate-like lymphocyte population that recognizes glycolipids presented on CD1d VSports手机版. Studies in mice have shown that these cells are heterogeneous and are capable of enacting diverse functions, and the composition of iNKT cell subsets can alter disease outcomes. In contrast, far less is known about how heterogeneity in human iNKT cells relates to disease. To address this, we used a high-dimensional, data-driven approach to devise a framework for parsing human iNKT heterogeneity. Our data revealed novel and previously described iNKT cell phenotypes with distinct functions. In particular, we found 2 phenotypes of interest: (1) a population with T helper 1 function that was increased with iNKT activation characterized by HLA-II+CD161- expression, and (2) a population with enhanced cytotoxic function characterized by CD4-CD94+ expression. These populations correlate with acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation and with new onset type 1 diabetes, respectively. Our study identifies human iNKT cell phenotypes associated with human disease that could aid in the development of biomarkers or therapeutics targeting iNKT cells. .
© 2020 by The American Society of Hematology V体育安卓版. .
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
V体育官网 - Figures

V体育平台登录 - References
-
- Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178(1):1-16. - VSports注册入口 - PMC - PubMed
-
- Gutierrez-Arcelus M, Teslovich N, Mola AR, et al. . Lymphocyte innateness defined by transcriptional states reflects a balance between proliferation and effector functions. Nat Commun. 2019;10(1):687. - VSports注册入口 - PMC - PubMed
-
- Lynch L, O’Shea D, Winter DC, Geoghegan J, Doherty DG, O’Farrelly C. Invariant NKT cells and CD1d(+) cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol. 2009;39(7):1893-1901. - PubMed
Publication types (VSports)
MeSH terms
- "VSports在线直播" Actions
- "V体育2025版" Actions
- "V体育安卓版" Actions
- "V体育官网" Actions
- VSports在线直播 - Actions
- "V体育平台登录" Actions
- V体育官网 - Actions
- "VSports注册入口" Actions
- Actions (VSports注册入口)
Substances (V体育官网)
Grants and funding
"V体育平台登录" LinkOut - more resources
Full Text Sources
Research Materials

