Vitamin D Receptor Inhibits NLRP3 Activation by Impeding Its BRCC3-Mediated Deubiquitination
- PMID: 31866999
- PMCID: PMC6904361 (V体育官网入口)
- DOI: V体育2025版 - 10.3389/fimmu.2019.02783
Vitamin D Receptor Inhibits NLRP3 Activation by Impeding Its BRCC3-Mediated Deubiquitination (VSports)
Abstract
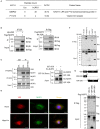
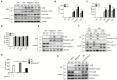
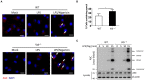
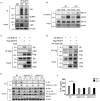
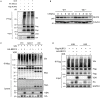
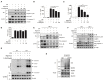
The NLRP3 inflammasome is a multiprotein oligomer responsible for activation of the inflammatory response by promoting the maturation and secretion of the pro-inflammatory cytokines IL-1β and IL-18. Dysregulation of this inflammasome has been linked to several autoimmune diseases, indicating that NLRP3 is tightly regulated to prevent aberrant activation. The regulation of NLRP3 activation remains unclear. Here, we report the identification of vitamin D receptor (VDR) as a negative regulator of NLRP3 oligomerization and activation. VDR can physically bind NLRP3 and block the association of NLRP3 with BRCC3. When BRCC3-mediated deubiquitination of NLRP3 is inhibited by VDR, NLRP3 activation is subsequently inhibited. In the absence of VDR, caspase-1 activation and IL-1β release are increased in response to LPS-induced inflammation or alum-induced peritoneal inflammation, indicating that VDR is a negative regulator of NLRP3 inflammasome activation in vivo. In addition, vitamin D negatively regulates the NLRP3 inflammasome via VDR signaling to effectively inhibit IL-1β secretion. These studies demonstrate that VDR signaling constrains NLRP3 inflammasome activation and might be a potential treatment target for NLRP3 inflammasome-related diseases VSports手机版. .
Keywords: BRCC3; NLRP3 inflammasome; VDR; cytokines; deubiquitinating. V体育安卓版.
Copyright © 2019 Rao, Chen, Wu, Xiao, Zhang, Wang, Fang, Zhang, Wang, Yang and Chen V体育ios版. .
Figures

References
-
- Broz P, Dixit VM. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol. (2016) 16:407–20. 10.1038/nri.2016.58 - DOI (V体育2025版) - PubMed
Publication types
- V体育官网入口 - Actions
MeSH terms
- "VSports注册入口" Actions
- "VSports手机版" Actions
- VSports在线直播 - Actions
- Actions (VSports在线直播)
- "VSports" Actions
- Actions (VSports app下载)
- Actions (V体育ios版)
- V体育安卓版 - Actions
- "V体育2025版" Actions
Substances
- V体育官网入口 - Actions
- Actions (VSports注册入口)
LinkOut - more resources (V体育2025版)
Full Text Sources (VSports注册入口)
Medical (V体育2025版)
"VSports" Molecular Biology Databases
Miscellaneous

