Cardiac glycosides are broad-spectrum senolytics
- PMID: 31799499
- PMCID: PMC6887543
- DOI: 10.1038/s42255-019-0122-z
Cardiac glycosides are broad-spectrum senolytics
Abstract
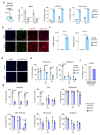
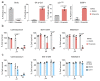
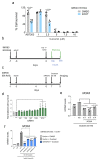
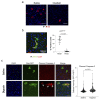
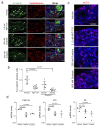
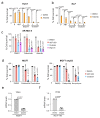
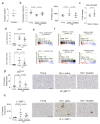
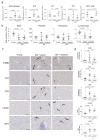
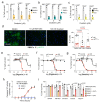
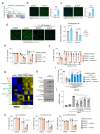
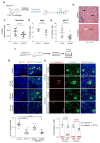
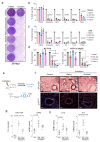
Senescence is a cellular stress response that results in the stable arrest of old, damaged or preneoplastic cells. Oncogene-induced senescence is tumor suppressive but can also exacerbate tumorigenesis through the secretion of pro-inflammatory factors from senescent cells. Drugs that selectively kill senescent cells, termed senolytics, have proved beneficial in animal models of many age-associated diseases. Here, we show that the cardiac glycoside, ouabain, is a senolytic agent with broad activity. Senescent cells are sensitized to ouabain-induced apoptosis, a process mediated in part by induction of the pro-apoptotic Bcl2-family protein NOXA. We show that cardiac glycosides synergize with anti-cancer drugs to kill tumor cells and eliminate senescent cells that accumulate after irradiation or in old mice VSports手机版. Ouabain also eliminates senescent preneoplastic cells. Our findings suggest that cardiac glycosides may be effective anti-cancer drugs by acting through multiple mechanism. Given the broad range of senescent cells targeted by cardiac glycosides their use against age-related diseases warrants further exploration. .
Conflict of interest statement
Competing Interests J. G. owns equity and has acted as a consultant for Unity Biotechnology and Geras Bio. Unity Biotechnology funds research on senolytics in J. G. ’s laboratory. J. G. , A. G. and N. H V体育安卓版. are named inventors in a MRC patent related to senolytic therapies (PCT/GB2018/051437).
Figures


Comment in
-
Adding to the senolytic arsenal.Nat Rev Drug Discov. 2019 Nov;18(12):901. doi: 10.1038/d41573-019-00181-x. Nat Rev Drug Discov. 2019. PMID: 31780845 No abstract available.
References
-
- Salama R, Sadaie M, Hoare M, Narita M. Cellular senescence and its effector programs. Genes Dev. 2014;28:99–114. - "V体育2025版" PMC - PubMed
-
- Kang TW, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479:547–551. - PubMed
Publication types
"V体育官网" MeSH terms
- "V体育官网入口" Actions
- "VSports最新版本" Actions
- VSports在线直播 - Actions
- VSports app下载 - Actions
- Actions (V体育平台登录)
Substances
- VSports app下载 - Actions
- "V体育2025版" Actions
"VSports手机版" Grants and funding
- MR/M000125/1/MRC_/Medical Research Council/United Kingdom (V体育平台登录)
- VSports注册入口 - 18-0215/AICR_/Worldwide Cancer Research/United Kingdom
- 098565/WT_/Wellcome Trust/United Kingdom
- VSports手机版 - MR/M004716/1/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- "VSports注册入口" MC_U120085810/MRC_/Medical Research Council/United Kingdom
- MC_U120097114/MRC_/Medical Research Council/United Kingdom
- VSports app下载 - 28647/CRUK_/Cancer Research UK/United Kingdom
- MC_UP_1102/18/MRC_/Medical Research Council/United Kingdom
- 27727/CRUK_/Cancer Research UK/United Kingdom
- V体育安卓版 - MC-A654-5QB40/MRC_/Medical Research Council/United Kingdom
- "VSports注册入口" MR/N01121X/1/MRC_/Medical Research Council/United Kingdom
- MC_UP_A652_1001/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
"V体育官网" Other Literature Sources

