V体育官网 - Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export
- PMID: 31735663
- PMCID: PMC8316835
- DOI: 10.1016/j.devcel.2019.10.007
Prominin2 Drives Ferroptosis Resistance by Stimulating Iron Export
Abstract
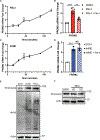
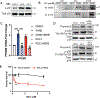
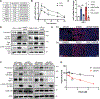
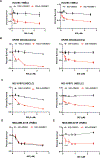
Ferroptosis, regulated cell death characterized by the iron-dependent accumulation of lethal lipid reactive oxygen species, contributes to tissue homeostasis and numerous pathologies, and it may be exploited for therapy. Cells differ in their sensitivity to ferroptosis, however, and a key challenge is to understand mechanisms that contribute to resistance. Using RNA-seq to identify genes that contribute to ferroptosis resistance, we discovered that pro-ferroptotic stimuli, including inhibition of the lipid hydroperoxidase GPX4 and detachment from the extracellular matrix, induce expression of prominin2, a pentaspanin protein implicated in regulation of lipid dynamics. Prominin2 facilitates ferroptosis resistance in mammary epithelial and breast carcinoma cells. Mechanistically, prominin2 promotes the formation of ferritin-containing multivesicular bodies (MVBs) and exosomes that transport iron out of the cell, inhibiting ferroptosis. These findings reveal that ferroptosis resistance can be driven by a prominin2-MVB-exosome-ferritin pathway and have broad implications for iron homeostasis, intracellular trafficking, and cancer VSports手机版. .
Keywords: GPX4; breast cancer; exosome; ferritin; ferroptosis; iron; mammary gland; multivesicular body; prominin2; therapy V体育安卓版. .
Copyright © 2019 Elsevier Inc V体育ios版. All rights reserved. .
"VSports注册入口" Conflict of interest statement
Figures

Comment in
-
Iron expulsion by exosomes drives ferroptosis resistance.Nat Rev Mol Cell Biol. 2020 Jan;21(1):4-5. doi: 10.1038/s41580-019-0195-2. Nat Rev Mol Cell Biol. 2020. PMID: 31748716 No abstract available.
-
Prominin-2 Suppresses Ferroptosis Sensitivity.Dev Cell. 2019 Dec 2;51(5):548-549. doi: 10.1016/j.devcel.2019.11.004. Dev Cell. 2019. PMID: 31794716
References
-
- Brown CW AJ, Goel HL, Mercurio AM (2017). The α6β4 integrin promotes resistance to ferroptosis. The Journal of cell biology 216, 4287–4297. - VSports注册入口 - PMC - PubMed
-
- Cella N C-UR, Montes GS, Hynes NE, Chammas R. (1996). The lysosomal-associated membrane protein LAMP-1 is a novel differentiation marker for HC11 mouse mammary epithelial cells. Differentiation 61, 113–120. - PubMed
-
- Dixon S.a.S., BR (2019). The Hallmarks of Ferroptosis. Annu Rev Cancer Biol 3, 35–54.
Publication types
- Actions (V体育官网)
MeSH terms
- "VSports最新版本" Actions
- Actions (V体育官网入口)
- VSports最新版本 - Actions
- Actions (V体育安卓版)
- V体育官网入口 - Actions
- Actions (V体育官网)
Substances
- V体育2025版 - Actions
- Actions (V体育平台登录)
VSports app下载 - Grants and funding
VSports手机版 - LinkOut - more resources
Full Text Sources
Other Literature Sources (VSports)
Medical

