Host-Microbe-Drug-Nutrient Screen Identifies Bacterial Effectors of Metformin Therapy
- PMID: 31474368
- PMCID: PMC6736778
- DOI: 10.1016/j.cell.2019.08.003
Host-Microbe-Drug-Nutrient Screen Identifies Bacterial Effectors of Metformin Therapy
Abstract
Metformin is the first-line therapy for treating type 2 diabetes and a promising anti-aging drug. We set out to address the fundamental question of how gut microbes and nutrition, key regulators of host physiology, affect the effects of metformin VSports手机版. Combining two tractable genetic models, the bacterium E. coli and the nematode C. elegans, we developed a high-throughput four-way screen to define the underlying host-microbe-drug-nutrient interactions. We show that microbes integrate cues from metformin and the diet through the phosphotransferase signaling pathway that converges on the transcriptional regulator Crp. A detailed experimental characterization of metformin effects downstream of Crp in combination with metabolic modeling of the microbiota in metformin-treated type 2 diabetic patients predicts the production of microbial agmatine, a regulator of metformin effects on host lipid metabolism and lifespan. Our high-throughput screening platform paves the way for identifying exploitable drug-nutrient-microbiome interactions to improve host health and longevity through targeted microbiome therapies. VIDEO ABSTRACT. .
Keywords: C V体育安卓版. elegans; CRP signaling; Drosophila; aging; diet; humans; metabolic modeling; metformin; microbiome; type-2 diabetes. .
Copyright © 2019 The Authors. Published by Elsevier Inc V体育ios版. All rights reserved. .
Conflict of interest statement
All authors declare no competing interests.
"V体育ios版" Figures
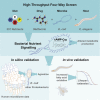
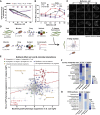
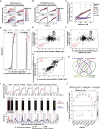
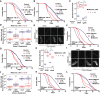

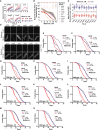
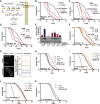

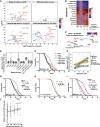

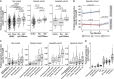


Comment in
-
VSports - Bacteria transmit metformin-associated lifespan extension.Nat Rev Endocrinol. 2020 Jan;16(1):9-10. doi: 10.1038/s41574-019-0278-3. Nat Rev Endocrinol. 2020. PMID: 31645681 No abstract available.
References
-
- Ackerman D., Gems D. Insulin/IGF-1 and hypoxia signaling act in concert to regulate iron homeostasis in Caenorhabditis elegans. PLoS Genet. 2012;8:e1002498. - "V体育2025版" PMC - PubMed
-
- Bauer P.V., Duca F.A., Waise T.M.Z., Rasmussen B.A., Abraham M.A., Dranse H.J., Puri A., O’Brien C.A., Lam T.K.T. Metformin Alters Upper Small Intestinal Microbiota that Impact a Glucose-SGLT1-Sensing Glucoregulatory Pathway. Cell Metab. 2018;27:101–117.e5. - PubMed
-
- Brandstetter B.R., Korfmann A., Kroke A., Becker N., Schulze M.B., Boeing H. Dietary habits in the German EPIC cohorts: food group intake estimated with the food frequency questionnaire. European Investigation into Cancer and Nutrition. Ann. Nutr. Metab. 1999;43:246–257. - PubMed
-
- Burkewitz K., Morantte I., Weir H.J.M., Yeo R., Zhang Y., Huynh F.K., Ilkayeva O.R., Hirschey M.D., Grant A.R., Mair W.B. Neuronal CRTC-1 governs systemic mitochondrial metabolism and lifespan via a catecholamine signal. Cell. 2015;160:842–855. - "VSports在线直播" PMC - PubMed
Publication types
MeSH terms
- "V体育ios版" Actions
- Actions (VSports app下载)
- "VSports手机版" Actions
- Actions (VSports手机版)
- "V体育ios版" Actions
- VSports手机版 - Actions
- "V体育官网入口" Actions
- Actions (V体育官网)
Substances
- "VSports注册入口" Actions
- Actions (V体育安卓版)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

