Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis
- PMID: 31316058
- PMCID: PMC6637122
- DOI: 10.1038/s41467-019-10991-7
Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis (VSports)
Abstract
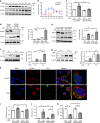
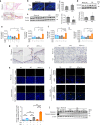
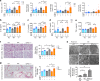
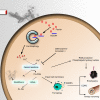
Ferroptosis is a necrotic form of regulated cell death (RCD) mediated by phospholipid peroxidation in association with free iron-mediated Fenton reactions. Disrupted iron homeostasis resulting in excessive oxidative stress has been implicated in the pathogenesis of chronic obstructive pulmonary disease (COPD). Here, we demonstrate the involvement of ferroptosis in COPD pathogenesis. Our in vivo and in vitro models show labile iron accumulation and enhanced lipid peroxidation with concomitant non-apoptotic cell death during cigarette smoke (CS) exposure, which are negatively regulated by GPx4 activity. Treatment with deferoxamine and ferrostatin-1, in addition to GPx4 knockdown, illuminate the role of ferroptosis in CS-treated lung epithelial cells. NCOA4-mediated ferritin selective autophagy (ferritinophagy) is initiated during ferritin degradation in response to CS treatment. CS exposure models, using both GPx4-deficient and overexpressing mice, clarify the pivotal role of GPx4-regulated cell death during COPD. These findings support a role for cigarette smoke-induced ferroptosis in the pathogenesis of COPD. VSports手机版.
Conflict of interest statement (VSports注册入口)
The authors declare no competing interests.
VSports app下载 - Figures


References
-
- Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. - "VSports在线直播" DOI - PubMed
Publication types
- V体育2025版 - Actions
MeSH terms
- Actions (VSports)
- Actions (V体育官网入口)
- "VSports最新版本" Actions
- VSports在线直播 - Actions
- VSports - Actions
- "V体育ios版" Actions
- V体育平台登录 - Actions
Substances
- Actions (VSports在线直播)
- "V体育2025版" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical (V体育2025版)
Molecular Biology Databases (V体育官网入口)

