"VSports在线直播" Docosahexaenoic acid inhibits both NLRP3 inflammasome assembly and JNK-mediated mature IL-1β secretion in 5-fluorouracil-treated MDSC: implication in cancer treatment
- PMID: 31217433
- PMCID: PMC6584690
- DOI: 10.1038/s41419-019-1723-x
Docosahexaenoic acid inhibits both NLRP3 inflammasome assembly and JNK-mediated mature IL-1β secretion in 5-fluorouracil-treated MDSC: implication in cancer treatment
Abstract
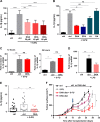
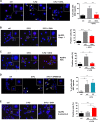
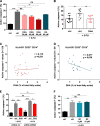
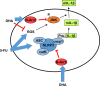
Limitation of 5-fluorouracil (5-FU) anticancer efficacy is due to IL-1β secretion by myeloid-derived suppressor cells (MDSC), according to a previous pre-clinical report VSports手机版. Release of mature IL-1β is a consequence of 5-FU-mediated NLRP3 activation and subsequent caspase-1 activity in MDSC. IL-1β sustains tumor growth recovery in 5-FU-treated mice. Docosahexaenoic acid (DHA) belongs to omega-3 fatty acid family and harbors both anticancer and anti-inflammatory properties, which could improve 5-FU chemotherapy. Here, we demonstrate that DHA inhibits 5-FU-induced IL-1β secretion and caspase-1 activity in a MDSC cell line (MSC-2). Accordingly, we showed that DHA-enriched diet reduces circulating IL-1β concentration and tumor recurrence in 5-FU-treated tumor-bearing mice. Treatment with 5-FU led to JNK activation through ROS production in MDSC. JNK inhibitor SP600125 as well as DHA-mediated JNK inactivation decreased IL-1β secretion. The repression of 5-FU-induced caspase-1 activity by DHA supplementation is partially due to β-arrestin-2-dependent inhibition of NLRP3 inflammasome activity but was independent of JNK pathway. Interestingly, we showed that DHA, through β-arrestin-2-mediated inhibition of JNK pathway, reduces V5-tagged mature IL-1β release induced by 5-FU, in MDSC stably overexpressing a V5-tagged mature IL-1β form. Finally, we found a negative correlation between DHA content in plasma and the induction of caspase-1 activity in HLA-DR- CD33+ CD15+ MDSC of patients treated with 5-FU-based chemotherapy, strongly suggesting that our data are clinical relevant. Together, these data provide new insights on the regulation of IL-1β secretion by DHA and on its potential benefit in 5-FU-based chemotherapy. .
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Prenen H, Vecchione L, Van Cutsem E. Role of targeted agents in metastatic colorectal cancer. Target. Oncol. 2013;8:83–96. doi: 10.1007/s11523-013-0281-x. - DOI (VSports在线直播) - PubMed
-
- Bronte V, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016;7:12150. doi: 10.1038/ncomms12150. - DOI (VSports在线直播) - PMC - PubMed
V体育官网入口 - Publication types
- "VSports手机版" Actions
MeSH terms (V体育官网入口)
- "VSports手机版" Actions
- "VSports" Actions
- Actions (V体育安卓版)
- VSports - Actions
- "V体育安卓版" Actions
- "VSports注册入口" Actions
- VSports在线直播 - Actions
- "V体育安卓版" Actions
- Actions (VSports手机版)
Substances
- V体育2025版 - Actions
- "VSports在线直播" Actions
LinkOut - more resources
Full Text Sources
"V体育平台登录" Research Materials

