Target engagement in an alzheimer trial: Crenezumab lowers amyloid β oligomers in cerebrospinal fluid
- PMID: 31168802
- PMCID: PMC6771589
- DOI: 10.1002/ana.25513
Target engagement in an alzheimer trial: Crenezumab lowers amyloid β oligomers in cerebrospinal fluid
Abstract
Objective: Oligomeric forms of amyloid β protein (oAβ) are believed to be principally responsible for neurotoxicity in Alzheimer disease (AD), but it is not known whether anti-Aβ antibodies are capable of lowering oAβ levels in humans VSports手机版. .
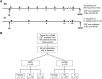
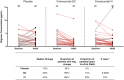
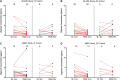
Methods: We developed an ultrasensitive immunoassay and used it to measure oAβ in cerebrospinal fluid (CSF) from 104 AD subjects participating in the ABBY and BLAZE phase 2 trials of the anti-Aβ antibody crenezumab. Patients received subcutaneous (SC) crenezumab (300mg) or placebo every 2 weeks, or intravenous (IV) crenezumab (15mg/kg) or placebo every 4 weeks for 68 weeks. Ninety-eight of the 104 patients had measurable baseline oAβ levels, and these were compared to levels at week 69 in placebo (n = 28), SC (n = 35), and IV (n = 35) treated patients. V体育安卓版.
Results: Among those receiving crenezumab, 89% of SC and 86% of IV patients had lower levels of oAβ at week 69 versus baseline V体育ios版. The difference in the proportion of patients with decreasing levels was significant for both treatment arms: p = 0. 0035 for SC and p = 0. 01 for IV crenezumab versus placebo. The median percentage change was -48% in the SC arm and -43% in the IV arm. No systematic change was observed in the placebo group, with a median change of -13% and equivalent portions with negative and positive change. .
Interpretation: Crenezumab lowered CSF oAβ levels in the large majority of treated patients tested. These results support engagement of the principal pathobiological target in AD and identify CSF oAβ as a novel pharmacodynamic biomarker for use in trials of anti-Aβ agents. ANN NEUROL 2019;86:215-224 VSports最新版本. .
© 2019 The Authors V体育平台登录. Annals of Neurology published by Wiley Periodicals, Inc. on behalf of American Neurological Association. .
Conflict of interest statement
V VSports注册入口. S. , C. R. , and T. B. are employees of Genentech, a member of the Roche Group. Crenezumab is under development by Roche. The other authors declare no conflicts of interest.
Figures



V体育官网 - References
-
- Cline EN, Bicca MA, Viola KL, Klein WL. The amyloid‐beta oligomer hypothesis: beginning of the third decade. J Alzheimers Dis 2018;64:S567–S610. - VSports手机版 - PMC - PubMed
Publication types
- VSports注册入口 - Actions
VSports手机版 - MeSH terms
- VSports注册入口 - Actions
- VSports最新版本 - Actions
- Actions (VSports注册入口)
- Actions (VSports)
- VSports在线直播 - Actions
- Actions (V体育官网入口)
Substances
- Actions (V体育官网)
- Actions (VSports)
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

