"V体育官网入口" EBV(LMP1)-induced metabolic reprogramming inhibits necroptosis through the hypermethylation of the RIP3 promoter
- PMID: 31131045
- PMCID: PMC6525991
- DOI: 10.7150/thno.30941
EBV(LMP1)-induced metabolic reprogramming inhibits necroptosis through the hypermethylation of the RIP3 promoter
"VSports最新版本" Abstract
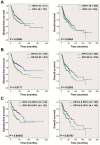
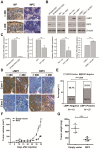
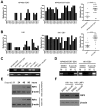
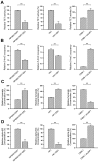
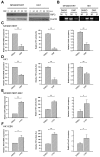
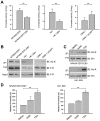
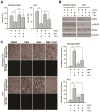
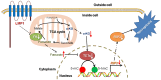
EBV infection is a recognized epigenetic driver of carcinogenesis. We previously showed that EBV could protect cancer cells from TNF-induced necroptosis. This study aims to explore the epigenetic mechanisms allowing cancer cells with EBV infection to escape from RIP3-dependent necroptosis. Methods: Data from the TCGA database were used to evaluate the prognostic value of RIP3 promoter methylation and its expression. Western blotting, real-time PCR, and immunochemistry were conducted to investigate the relationship between LMP1 and RIP3 in cell lines and NPC tissues. BSP, MSP and hMeDIP assays were used to examine the methylation level. Induction of necroptosis was detected by cell viability assay, p-MLKL, and Sytox Green staining. Results: RIP3 promoter hypermethylation is an independent prognostic factor of poorer disease-free and overall survival in HNSCC patients, respectively. RIP3 is down-regulated in NPC (a subtype of HNSCC). EBV(LMP1) suppresses RIP3 expression by hypermethylation of the RIP3 promoter. RIP3 protein expression was inversely correlated with LMP1 expression in NPC tissues. Restoring RIP3 expression in EBV(LMP1)-positive cells inhibits xenograft tumor growth VSports手机版. The accumulation of fumarate and reduction of α-KG in EBV(LMP1)-positive cells led to RIP3 silencing due to the inactivation of TETs. Decreased FH activity caused fumarate accumulation, which might be associated with its acetylation. Incubating cells with fumarate protected NPC cells from TNF-induced necroptosis. Conclusion: These results demonstrate a pathway by which EBV(LMP1)-associated metabolite changes inhibited necroptosis signaling by DNA methylation, and shed light on the mechanism underlying EBV-related carcinogenesis, which may provide new options for cancer diagnosis and therapy. .
Keywords: Epstein-Barr virus; Fumarate V体育安卓版. ; Nasopharyngeal carcinoma; Necroptosis; Receptor-interacting protein 3. .
Conflict of interest statement (V体育官网)
Competing Interests: The authors have declared that no competing interest exists.
"VSports注册入口" Figures
VSports app下载 - References
-
- He S, Wang L, Miao L, Wang T, Du F, Zhao L. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–11. - PubMed
-
- Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–6. - PubMed
-
- Koo GB, Morgan MJ, Lee DG, Kim WJ, Yoon JH, Koo JS. et al. Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res. 2015;25:707–25. - V体育2025版 - PMC - PubMed
-
- Danthi P. Viruses and the Diversity of Cell Death. Annu Rev Virol. 2016;3:533–53. - PubMed
"V体育2025版" Publication types
MeSH terms
- VSports app下载 - Actions
- Actions (VSports在线直播)
- "VSports最新版本" Actions
- Actions (VSports最新版本)
- "VSports注册入口" Actions
- VSports在线直播 - Actions
- "V体育ios版" Actions
- "V体育安卓版" Actions
- "V体育官网" Actions
- "V体育平台登录" Actions
- Actions (V体育安卓版)
- VSports最新版本 - Actions
- "VSports最新版本" Actions
- VSports app下载 - Actions
- VSports在线直播 - Actions
- "V体育ios版" Actions
- V体育安卓版 - Actions
- "VSports app下载" Actions
- V体育安卓版 - Actions
V体育官网入口 - Substances
- Actions (V体育官网入口)
- "VSports" Actions
- V体育2025版 - Actions
- V体育2025版 - Actions
- VSports在线直播 - Actions
LinkOut - more resources
Full Text Sources
V体育安卓版 - Other Literature Sources
Medical
Research Materials
V体育官网入口 - Miscellaneous

