A microbiota-generated bile salt induces biofilm formation in Clostridium difficile (V体育2025版)
- PMID: 31098293
- PMCID: PMC6509328
- DOI: 10.1038/s41522-019-0087-4
A microbiota-generated bile salt induces biofilm formation in Clostridium difficile
Abstract
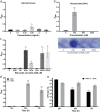
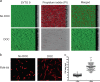
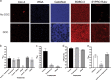
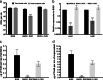
Clostridium difficile is a major cause of nosocomial infections. Bacterial persistence in the gut is responsible for infection relapse; sporulation and other unidentified mechanisms contribute to this process. Intestinal bile salts cholate and deoxycholate stimulate spore germination, while deoxycholate kills vegetative cells VSports手机版. Here, we report that sub-lethal concentrations of deoxycholate stimulate biofilm formation, which protects C. difficile from antimicrobial compounds. The biofilm matrix is composed of extracellular DNA and proteinaceous factors that promote biofilm stability. Transcriptomic analysis indicates that deoxycholate induces metabolic pathways and cell envelope reorganization, and represses toxin and spore production. In support of the transcriptomic analysis, we show that global metabolic regulators and an uncharacterized lipoprotein contribute to deoxycholate-induced biofilm formation. Finally, Clostridium scindens enhances biofilm formation of C. difficile by converting cholate into deoxycholate. Together, our results suggest that deoxycholate is an intestinal signal that induces C. difficile persistence and may increase the risk of relapse. .
Keywords: Bacteriology; Biofilms; Pathogens V体育安卓版. .
Conflict of interest statement
The authors declare no competing interests.
Figures
References (V体育官网)
-
- Figueroa I, et al. Relapse versus reinfection: recurrent Clostridium difficile infection following treatment with fidaxomicin or vancomycin. Clin. Infect. Dis. 2012;55:S104–S109. doi: 10.1093/cid/cis357. - "V体育平台登录" DOI - PMC - PubMed
-
- Janoir C. Virulence factors of Clostridium difficile and their role during infection. Anaerobe. 2016;37:13–24. doi: 10.1016/j.anaerobe.2015.10.009. - V体育平台登录 - DOI - PubMed
-
- Sorg JA, Sonenshein AL. Bile salts and glycine as cogerminants for Clostridium difficile spores. J. Bacteriol. 2008;190:2505–2512. doi: 10.1128/JB.01765-07. - DOI (VSports在线直播) - PMC - PubMed
"VSports app下载" Publication types
- "V体育官网入口" Actions
MeSH terms (VSports app下载)
- Actions (V体育官网)
- "VSports app下载" Actions
Substances
- Actions (VSports)
- V体育2025版 - Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

