Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation
- PMID: 30860577
- PMCID: PMC6934151
- DOI: 10.1093/jmcb/mjz020
"V体育官网入口" Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation
V体育官网 - Abstract
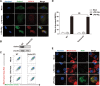
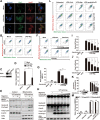
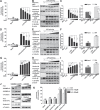
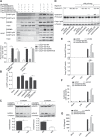
Disrupted mitochondrial membrane potential (MMP) and reactive oxygen species (ROS) generation are often associated with macrophage pyroptosis. It remains unclear how these forms of mitochondrial dysfunction relate to inflammasome activation and gasdermin-D (Gsdmd) cleavage, two central steps of the pyroptotic process. Here, we also found MMP collapse and ROS generation induced by Nlrp3 inflammasome activation as previous studies reported. The elimination of ROS alleviated the cleavage of Gsdmd, suggesting that Gsdmd cleavage occurs downstream of ROS release. Consistent with this result, hydrogen peroxide treatment augmented the cleavage of Gsdmd by caspase-1. Indeed, four amino acid residues of Gsdmd were oxidized under oxidative stress in macrophages. The efficiency of Gsdmd cleavage by inflammatory caspase-1 was dramatically reduced when oxidative modification was blocked by mutation of these amino acid residues. These results demonstrate that Gsdmd oxidation serves as a de novo mechanism by which mitochondrial ROS promote Nlrp3 inflammasome-dependent pyroptotic cell death VSports手机版. .
Keywords: Nlrp3; ROS; gasdermin-D; mitochondria; oxidation V体育安卓版. .
© The Author(s) (2019) V体育ios版. Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, IBCB, SIBS, CAS. .
Figures

V体育官网 - References
-
- Agostini L., Martinon F., Burns K., et al. (2004). NALP3 forms an IL-1β processing inflammasome with increased activity in Muckle–Wells autoinflammatory disorder. Immunity 20, 319–325. - PubMed (V体育官网入口)
-
- Ahmad S., Khan H., Shahab U. et al. (2017). Protein oxidation: an overview of metabolism of sulphur containing amino acid, cysteine. Front. Biosci. 9, 71–87. - PubMed
-
- Bauernfeind F., Bartok E., Rieger A., et al. (2011). Cutting edge: reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 187, 613–617. - V体育ios版 - PMC - PubMed
Publication types
VSports注册入口 - MeSH terms
- Actions (VSports手机版)
- Actions (VSports注册入口)
- "V体育2025版" Actions
- Actions (V体育2025版)
- Actions (V体育平台登录)
- Actions (VSports最新版本)
- "V体育官网" Actions
- "VSports手机版" Actions
Substances
- VSports app下载 - Actions

