A major role for ferroptosis in VSports手机版 - Mycobacterium tuberculosis-induced cell death and tissue necrosis
- PMID: 30787033
- PMCID: PMC6400546
- DOI: 10.1084/jem.20181776
A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis
Abstract
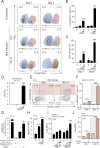
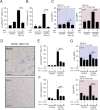
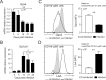
Necrotic cell death during Mycobacterium tuberculosis (Mtb) infection is considered host detrimental since it facilitates mycobacterial spread. Ferroptosis is a type of regulated necrosis induced by accumulation of free iron and toxic lipid peroxides. We observed that Mtb-induced macrophage necrosis is associated with reduced levels of glutathione and glutathione peroxidase-4 (Gpx4), along with increased free iron, mitochondrial superoxide, and lipid peroxidation, all of which are important hallmarks of ferroptosis. Moreover, necrotic cell death in Mtb-infected macrophage cultures was suppressed by ferrostatin-1 (Fer-1), a well-characterized ferroptosis inhibitor, as well as by iron chelation. Additional experiments in vivo revealed that pulmonary necrosis in acutely infected mice is associated with reduced Gpx4 expression as well as increased lipid peroxidation and is likewise suppressed by Fer-1 treatment. Importantly, Fer-1-treated infected animals also exhibited marked reductions in bacterial load. Together, these findings implicate ferroptosis as a major mechanism of necrosis in Mtb infection and as a target for host-directed therapy of tuberculosis. VSports手机版.
This is a work of the U V体育安卓版. S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply. .
Figures


Comment in
-
Die another way: Ferroptosis drives tuberculosis pathology.J Exp Med. 2019 Mar 4;216(3):471-473. doi: 10.1084/jem.20190038. Epub 2019 Feb 20. J Exp Med. 2019. PMID: 30787032 Free PMC article.
References
-
- Amaral E.P., Conceição E.L., Costa D.L., Rocha M.S., Marinho J.M., Cordeiro-Santos M., D’Império-Lima M.R., Barbosa T., Sher A., and Andrade B.B.. 2016b N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiol. 16:251 10.1186/s12866-016-0872-7 - DOI - PMC - PubMed
-
- Andrade B.B., Pavan Kumar N., Mayer-Barber K.D., Barber D.L., Sridhar R., Rekha V.V., Jawahar M.S., Nutman T.B., Sher A., and Babu S.. 2013. Plasma heme oxygenase-1 levels distinguish latent or successfully treated human tuberculosis from active disease. PLoS One. 8:e62618 10.1371/journal.pone.0062618 - DOI - PMC - PubMed
Publication types
- Actions (VSports app下载)
MeSH terms
- V体育平台登录 - Actions
- VSports最新版本 - Actions
- "VSports在线直播" Actions
- "VSports app下载" Actions
- VSports注册入口 - Actions
- VSports - Actions
Substances
- "VSports" Actions
- "VSports最新版本" Actions
- "VSports注册入口" Actions
LinkOut - more resources
Full Text Sources
Medical
V体育ios版 - Molecular Biology Databases

