VSports注册入口 - Modulation of Intestinal Epithelial Permeability by Plasma from Patients with Crohn's Disease in a Three-dimensional Cell Culture Model
- PMID: 30765731
- PMCID: PMC6375954
- DOI: VSports - 10.1038/s41598-018-38322-8
V体育ios版 - Modulation of Intestinal Epithelial Permeability by Plasma from Patients with Crohn's Disease in a Three-dimensional Cell Culture Model
Abstract
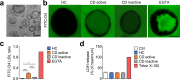
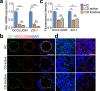
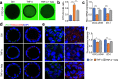
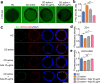
Intestinal epithelial barrier is affected by multiple factors, such as tumour necrosis factor-α (TNF-α). Plasma concentration of TNF-α is higher in patients with Crohn's disease (CD) than healthy controls (HC) and correlates positively with disease activity VSports手机版. This study aimed to determine the effect of plasma from active, inactive CD patients on intestinal barrier function and to investigate the underlying mechanism. Plasma samples were collected from CD patients and HC. 3D Caco-2 cysts were treated with plasma or TNF-α, with or without pre-incubation of adalimumab (a monoclonal antibody that antagonizes TNF-α) or JNK inhibitor SP600125. The results demonstrated that exposure of the cysts to plasma from CD patients resulted in enhanced paracellular permeability in a disease activity-dependent manner. Compared to HC, active CD plasma decreased ZO-1 and OCCLUDIN expression on mRNA and protein levels, and led to an increased JNK phosphorylation. Pre-incubation with adalimumab or SP600125 ameliorated TJ disruption and barrier dysfunction induced by plasma from CD patients. These results indicate that plasma from CD patients is able to induce epithelial barrier disruption, in part through TNF-α induced TJs modulation. The data also demonstrate an involvement of MAPK pathway, in particular the JNK isoform, in CD patient plasma-induced barrier dysfunction. .
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Bernklev T, et al. Health-related quality of life in patients with inflammatory bowel disease measured with the short form-36: psychometric assessments and a comparison with general population norms. Inflammatory bowel diseases. 2005;11:909–918. doi: 10.1097/01.mib.0000179467.01748.99. - DOI - PubMed
-
- Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20:91–99. doi: 10.3748/wjg.v20.i1.91. - "VSports注册入口" DOI - PMC - PubMed
-
- Braegger CP, Nicholls S, Murch SH, Stephens S, MacDonald TT. Tumour necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89–91. doi: 10.1016/0140-6736(92)90999-J. - "VSports app下载" DOI - PubMed
-
- Hagel AF, et al. Plasma histamine and tumour necrosis factor-alpha levels in Crohn’s disease and ulcerative colitis at various stages of disease. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. 2015;66:549–556. - PubMed (V体育安卓版)
"V体育平台登录" Publication types
MeSH terms
- V体育官网入口 - Actions
- "VSports最新版本" Actions
- "VSports注册入口" Actions
- Actions (VSports在线直播)
- Actions (V体育官网入口)
- V体育官网 - Actions
- V体育2025版 - Actions
- "VSports手机版" Actions
- "V体育安卓版" Actions
Substances
- Actions (VSports)
- "V体育2025版" Actions
- "VSports在线直播" Actions
- "V体育安卓版" Actions
- Actions (VSports)
"V体育官网" LinkOut - more resources
Full Text Sources
Research Materials

