Inhibition of PTGS1 promotes osteogenic differentiation of adipose-derived stem cells by suppressing NF-kB signaling
- PMID: 30760327
- PMCID: "VSports" PMC6375160
- DOI: 10.1186/s13287-019-1167-3
Inhibition of PTGS1 promotes osteogenic differentiation of adipose-derived stem cells by suppressing NF-kB signaling
Abstract
Background: Tissue inflammation is an important problem in the field of human adipose-derived stem cell (ASC)-based therapeutic bone regeneration. Many studies indicate that inflammatory cytokines are disadvantageous for osteogenic differentiation and bone formation. Therefore, overcoming inflammation would be greatly beneficial in promoting ASC-mediated bone regeneration. The present study aims to investigate the potential anti-inflammatory role of Prostaglandin G/H synthase 1 (PTGS1) during the osteogenic differentiation of ASCs. VSports手机版.
Methods: We performed TNFα treatment to investigate the response of PTGS1 to inflammation V体育安卓版. Loss- and gain-of-function experiments were applied to investigate the function of PTGS1 in the osteogenic differentiation of ASCs ex vivo and in vivo. Western blot and confocal analyses were used to determine the molecular mechanism of PTGS1-regulated osteogenic differentiation. .
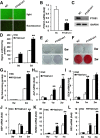
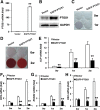
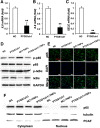
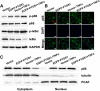
Results: Our work demonstrates that PTGS1 expression is significantly increased upon inflammatory cytokine treatment. Both ex vivo and in vivo studies indicate that PTGS1 is required for the osteogenic differentiation of ASCs. Mechanistically, we show that PTGS1 regulates osteogenesis of ASCs via modulating the NF-κB signaling pathway V体育ios版. .
Conclusions: Collectively, this work confirms that the PTGS1-NF-κB signaling pathway is a novel molecular target for ASC-mediated regenerative medicine. VSports最新版本.
Keywords: ASCs; NF-κB; Osteogenic differentiation; PTGS1. V体育平台登录.
Conflict of interest statement
Ethics approval and consent to participate
This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health VSports注册入口. The protocol was approved by the Institutional Animal Care and Use Committee of the Peking University Health Science Center (approval no. LA2014233). All surgeries were performed under anesthesia, and all efforts were made to minimize animal suffering.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Ito K, Yamada Y, Nagasaka T, Baba S, Ueda M. Osteogenic potential of injectable tissue-engineered bone: a comparison among autogenous bone, bone substitute (Bio-Oss), platelet-rich plasma, and tissue-engineered bone with respect to their mechanical properties and histological findings. J Biomed Mater Res A. 2005;73:63–67. doi: 10.1002/jbm.a.30248. - DOI (VSports注册入口) - PubMed
Publication types
MeSH terms
- Actions (VSports)
- Actions (V体育ios版)
- V体育2025版 - Actions
- "VSports" Actions
- Actions (V体育官网)
- "V体育平台登录" Actions
- Actions (V体育官网)
Substances
- V体育平台登录 - Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

