VSports手机版 - Single-Cell Transcriptomics of Regulatory T Cells Reveals Trajectories of Tissue Adaptation
- PMID: 30737144
- PMCID: PMC6382439
- DOI: 10.1016/j.immuni.2019.01.001
Single-Cell Transcriptomics of Regulatory T Cells Reveals Trajectories of Tissue Adaptation (VSports在线直播)
"V体育ios版" Abstract
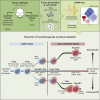
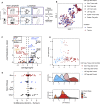
Non-lymphoid tissues (NLTs) harbor a pool of adaptive immune cells with largely unexplored phenotype and development. We used single-cell RNA-seq to characterize 35,000 CD4+ regulatory (Treg) and memory (Tmem) T cells in mouse skin and colon, their respective draining lymph nodes (LNs) and spleen. In these tissues, we identified Treg cell subpopulations with distinct degrees of NLT phenotype. Subpopulation pseudotime ordering and gene kinetics were consistent in recruitment to skin and colon, yet the initial NLT-priming in LNs and the final stages of NLT functional adaptation reflected tissue-specific differences. Predicted kinetics were recapitulated using an in vivo melanoma-induction model, validating key regulators and receptors. Finally, we profiled human blood and NLT Treg and Tmem cells, and identified cross-mammalian conserved tissue signatures. In summary, we describe the relationship between Treg cell heterogeneity and recruitment to NLTs through the combined use of computational prediction and in vivo validation VSports手机版. .
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved V体育安卓版. .
Figures




References
-
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. - PubMed
-
- Bollrath J., Powrie F.M. Controlling the frontier: regulatory T-cells and intestinal homeostasis. Semin. Immunol. 2013;25:352–357. - PubMed
-
- Campbell D.J., Koch M.A. Phenotypical and functional specialization of FOXP3+ regulatory T cells. Nat. Rev. Immunol. 2011;11:119–130. - PMC (VSports app下载) - PubMed
-
- Campbell K.R., Yau C. switchde: inference of switch-like differential expression along single-cell trajectories. Bioinformatics. 2017;33:1241–1242. - "VSports最新版本" PMC - PubMed
Publication types
MeSH terms
- V体育安卓版 - Actions
- "V体育平台登录" Actions
- Actions (V体育平台登录)
- VSports手机版 - Actions
- VSports手机版 - Actions
- "V体育安卓版" Actions
- Actions (VSports)
- "V体育安卓版" Actions
- "V体育2025版" Actions
- V体育官网 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
"V体育ios版" Other Literature Sources
"VSports手机版" Molecular Biology Databases
Research Materials

