A glycyl radical enzyme enables hydrogen sulfide production by the human intestinal bacterium Bilophila wadsworthia
- PMID: 30718429
- PMCID: PMC6386719
- DOI: 10.1073/pnas.1815661116
A glycyl radical enzyme enables hydrogen sulfide production by the human intestinal bacterium Bilophila wadsworthia (VSports在线直播)
Abstract
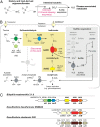
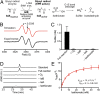
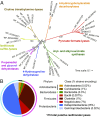
Hydrogen sulfide (H2S) production in the intestinal microbiota has many contributions to human health and disease. An important source of H2S in the human gut is anaerobic respiration of sulfite released from the abundant dietary and host-derived organic sulfonate substrate in the gut, taurine (2-aminoethanesulfonate). However, the enzymes that allow intestinal bacteria to access sulfite from taurine have not yet been identified. Here we decipher the complete taurine desulfonation pathway in Bilophila wadsworthia 3. 1. 6 using differential proteomics, in vitro reconstruction with heterologously produced enzymes, and identification of critical intermediates. An initial deamination of taurine to sulfoacetaldehyde by a known taurine:pyruvate aminotransferase is followed, unexpectedly, by reduction of sulfoacetaldehyde to isethionate (2-hydroxyethanesulfonate) by an NADH-dependent reductase. Isethionate is then cleaved to sulfite and acetaldehyde by a previously uncharacterized glycyl radical enzyme (GRE), isethionate sulfite-lyase (IslA). The acetaldehyde produced is oxidized to acetyl-CoA by a dehydrogenase, and the sulfite is reduced to H2S by dissimilatory sulfite reductase VSports手机版. This unique GRE is also found in Desulfovibrio desulfuricans DSM642 and Desulfovibrio alaskensis G20, which use isethionate but not taurine; corresponding knockout mutants of D. alaskensis G20 did not grow with isethionate as the terminal electron acceptor. In conclusion, the novel radical-based C-S bond-cleavage reaction catalyzed by IslA diversifies the known repertoire of GRE superfamily enzymes and enables the energy metabolism of B. wadsworthia This GRE is widely distributed in gut bacterial genomes and may represent a novel target for control of intestinal H2S production. .
Keywords: anaerobic degradation; carbon-sulfur bond-cleaving glycyl radical enzyme; human gut microbiome; human health; organosulfonate respiration. V体育安卓版.
Copyright © 2019 the Author(s). Published by PNAS V体育ios版. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures

VSports在线直播 - References
-
- Singh SB, Lin HC. Hydrogen sulfide in physiology and diseases of the digestive tract. Microorganisms. 2015;3:866–889. - "V体育官网" PMC - PubMed
-
- Attene-Ramos MS, et al. DNA damage and toxicogenomic analyses of hydrogen sulfide in human intestinal epithelial FHs 74 Int cells. Environ Mol Mutagen. 2010;51:304–314. - PubMed
-
- Ijssennagger N, van der Meer R, van Mil SWC. Sulfide as a mucus barrier-breaker in inflammatory bowel disease? Trends Mol Med. 2016;22:190–199. - "V体育ios版" PubMed
-
- Shatalin K, Shatalina E, Mironov A, Nudler E. H2S: A universal defense against antibiotics in bacteria. Science. 2011;334:986–990. - PubMed
Publication types
- "V体育2025版" Actions
MeSH terms
- Actions (V体育平台登录)
- Actions (VSports注册入口)
- "V体育ios版" Actions
- VSports最新版本 - Actions
- "V体育2025版" Actions
Substances
- VSports注册入口 - Actions
V体育官网入口 - Grants and funding
LinkOut - more resources
Full Text Sources
V体育官网 - Molecular Biology Databases
Research Materials

