The Deubiquitylase OTUB1 Mediates Ferroptosis via Stabilization of SLC7A11
- PMID: 30709928
- PMCID: PMC6467774
- DOI: 10.1158/0008-5472.CAN-18-3037
The Deubiquitylase OTUB1 Mediates Ferroptosis via Stabilization of SLC7A11
Abstract
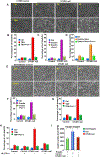
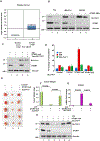
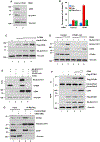
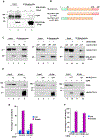
Although cell-cycle arrest, senescence, and apoptosis are established mechanisms of tumor suppression, accumulating evidence reveals that ferroptosis, an iron-dependent, nonapoptotic form of cell death, represents a new regulatory pathway in suppressing tumor development. Ferroptosis is triggered by lipid peroxidation and is tightly regulated by SLC7A11, a key component of the cystine-glutamate antiporter. Although many studies demonstrate the importance of transcriptional regulation of SLC7A11 in ferroptotic responses, it remains largely unknown how the stability of SLC7A11 is controlled in human cancers. In this study, we utilized biochemial purification to identify the ubiquitin hydrolase OTUB1 as a key factor in modulating SLC7A11 stability. OTUB1 directly interacted with and stabilized SLC7A11; conversely, OTUB1 knockdown diminished SLC7A11 levels in cancer cells. OTUB1 was overexpressed in human cancers, and inactivation of OTUB1 destabilized SLC7A11 and led to growth suppression of tumor xenografts in mice, which was associated with reduced activation of ferroptosis. Notably, overexpression of the cancer stem cell marker CD44 enhanced the stability of SLC7A11 by promoting the interaction between SLC7A11 and OTUB1; depletion of CD44 partially abrogated this interaction VSports手机版. CD44 expression suppressed ferroptosis in cancer cells in an OTUB1-dependent manner. Together, these results show that OTUB1 plays an essential role in controlling the stability of SLC7A11 and the CD44-mediated effects on ferroptosis in human cancers. SIGNIFICANCE: This study identifies OTUB1 as a key regulator of ferroptosis and implicates it as a potential target in cancer therapy. See related commentary by Gan, p. 1749. .
©2019 American Association for Cancer Research V体育安卓版. .
"V体育官网入口" Conflict of interest statement
There are no potential conflicts of interest disclosed.
Figures


Comment in
-
DUBbing Ferroptosis in Cancer Cells.Cancer Res. 2019 Apr 15;79(8):1749-1750. doi: 10.1158/0008-5472.CAN-19-0487. Cancer Res. 2019. PMID: 30987975 Free PMC article.
References
-
- Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, et al. Ferroptosis: process and function. Cell death and differentiation 2016;23:369–79 - "V体育官网" PMC - PubMed
-
- Conrad M, Sato H. The oxidative stress-inducible cystine/glutamate antiporter, system × (c) (−): cystine supplier and beyond. Amino acids 2012;42:231–46 - PubMed
MeSH terms
- Actions (V体育安卓版)
- "V体育官网入口" Actions
- Actions (VSports最新版本)
- VSports注册入口 - Actions
- V体育安卓版 - Actions
Substances
- V体育官网 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

